ChemVizOverview |
Overview of the Born-Oppenheimer Approximation |
| Born Oppenheimer Approximation Overview Materials
Readings Lab Activities Support Materials Related Links Developers' Tools
|
How Much Do I Already Know?Key Points
OverviewIn order to be able to solve Schroëdinger's equation for any system larger than an atom with one electron, various approximations need to be made. Each of these approximations contributes its own inaccuracies to the final result, which must be accounted for in reporting results. One of the more important approximations used is the Born-Oppenheimer approximation.The Born-Oppenheimer approximation is the first of several approximations made when trying to solve Schroëdinger's equation for more complex systems than one or two electrons. It separates electron and nuclear motion based on the idea that nuclear mass is so much larger than electron mass that the nuclei are basically "fixed" particles. Electrons react instanteously to changes in the position of the nuclei. This equation shows the full Hamiltonian for a molecular system.
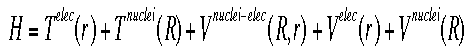 Shown below is the equation for the "electronic" Hamiltonian (one that neglects the kinetic energy term for the nuclei).
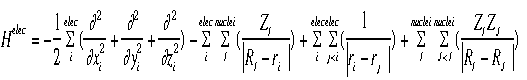 Below is the Hamiltonian used in the Schroëdinger equation describing the motion of electrons in a field of fixed nuclei, shown in the first equation.
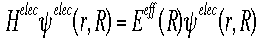 Solving this equation for the electronic wavefunction will produce Eeff, the effective nuclear potential function. 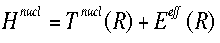 Eeff is used as the potential for the nuclear Hamiltonian. This Hamiltonian is used for nuclear motion, describing the vibrational, rotational, and translational states of the nuclei. |
in cooperation with the
National Center for Supercomputing Applications
© Copyright 1999-2000 The Shodor Education Foundation, Inc.