ChemVizLab Activities |
Background Reading for Ionization Energy: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization Energy
Students
TeachersReadings Lab Activities Support Materials Related Links Developers' Tools
|
Key PointsBasic: As stated in earlier reading, ionization energies (IE) have to do with things called ions. Ions are atoms which have gained or lost electrons. The ionization energy is the amount of energy it takes to detach one electron from a neutral atom. Some elements actually have several ionization energies. When this is the case, we refer to them as the "first ionization energy" or 'I', "second ionization energy" or 'I2', and so on. Notice that the energy variable follows Ii where i is the orbital from which the electron is lost. Ionization is endothermic meaning that the atom or molecule increases its internal energy (takes energy from an outside source). The equation for the first ionization energy is shown below:
The equation for the second ionization energy is:
Ionization energy values are typically very high and follow trends throughout the periodic table. The IE increase from bottom to top and left to right in the periodic table.
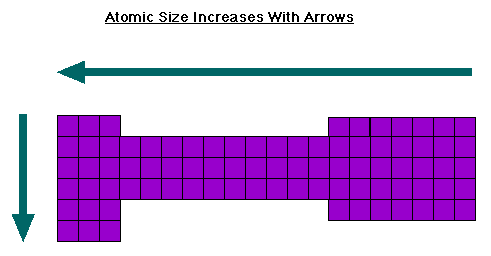
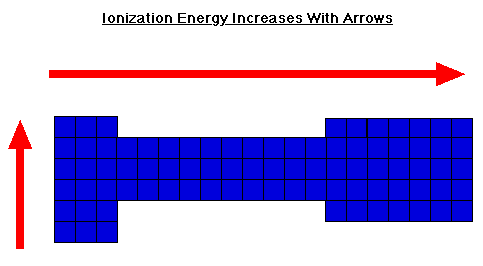 Below is a diagram showing the directions atomic size increase over the periodic table. As you can see, the IE and atomic size increase in opposite directions. This should make sense because as the atom gets smaller, the valence electrons become closer to the nucleus. This means the attractive force holding the electron is stronger and it takes more energy to pull the electron off.
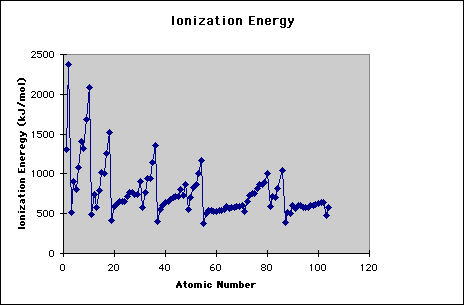
 Another trend is found when looking at the first IE of each atom. Below you can see the pattern when the IE is graphed against the atomic number. When looking at this diagram, you should notice that the increasing trend for atoms going horizontally across the periodic table is not absolute. This means that when you are looking at two atoms, the one furthest right does not always have the higher IE. However, there is an overall trend that shows an increasing IE the further right you are in the periodic table. These inconsistencies are attributed to the actual type of orbital the electron is being removed from. For instance, a 2p orbital has a higher energy than a 2s.
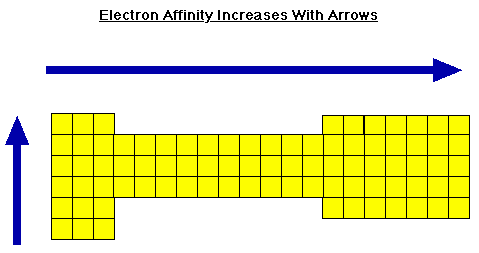
 The ionization energy of an atom is equal to the amount of energy given off when an electron is added to an atom. When an electron is added to an atom, we call the energy given off the electron affinity (EA). So, IE=EA. For most atoms, the initial electron affinity is exothermic meaning energy is given off. However, when you try to add a second, third, etc. electron you are working with an already negative ion. Thus, it takes a greater energy to add the extra electron and therefore the EAs after this first are normally endothermic. A good approximation of electron affinity is the energy of the LUMO (lowest unoccupied molecular orbital). Electron affinities follow the same trends as the ionization energy across the periodic table as seen below.
 The first of two main methods which scientists use to calculate the ionization energy is the Subtraction Method. This method entails some experimentation. You must first find the energy value of the ion you are looking for. Then subtract the energy value of the neutral atom. This difference is the ionization energy for that ion. Your answers can easily be checked against literature values published in most chemistry books. One such abbreviated table is shown below.
Ionization Energies in kJ/mol
Advanced: So far, we have discussed the basics of ionization energy. Now we want to look at the more advanced methods of solving for it. Two key characteristics of an atom or molecule that are very important to scientists are the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Together, these two orbitals are called the frontier orbitals. The HOMO can be found by locating the outer most orbital containing an electron. The LUMO then is the first orbital that does not contain an electron. See the diagram below:
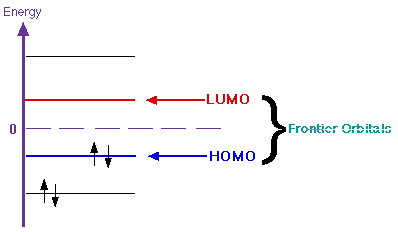 The frontier orbitals are important for many reasons. Earlier we talked about the first method for calculating the ionization energy, subtraction. The second method is called Koopman's Theory. This method involves the HOMO. It states that the ionization energy of an atom or molecule is equal to the energy of the orbital from which the electron is ejected. This means that the ionization energy is equal to the HOMO energy. The formal equation is shown below.
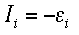 Where i is the orbital from which the electron is ejected (the HOMO) and Ei is the energy of that orbital. This formula is only exact for Hydrogenic atoms. For other atoms, it is only an approximation because it does not take into account electron movement after an electron has been removed. Instead, it assumes all electrons stay in the same orbitals once ionization has occured. The frontier orbitals are also important because they are key factors in the amount of energy needed to add or remove electrons in a molecule. When an electron is removed from one atom/molecule it is taken from the HOMO and when an electron is added, it is added to the LUMO. Knowing the frontier orbitals also gives scientists some insight into various aspects of the molecule such as the electronegativity, hardness and aromaticity.
|
in cooperation with the
National Center for Supercomputing Applications
© Copyright 1999-2000 The Shodor Education Foundation, Inc.