ChemVizLab Activities |
Hardness (and Softness): | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization Energy
Students
TeachersReadings Lab Activities Support Materials Related Links Developers' Tools
|
Hardness and softness are terms used to help describe various
elements' likeliness to bond. The periodic table is split into two main types
of atoms, polarizable and unpolarizable. (Some of you may need to review polarity.) Polarizable atoms form strong bonds
with other polarizable atoms whereas unpolarizable atoms form strong bonds
with other unpolarizable atoms. Eventually, polarizable atoms have become
known as "soft" atoms and unpolarizable atom as "hard". This
means that when soft-soft atoms bond it is normally a covalent bond and when hard-hard atoms
bond it is normally ionic. A chemist by the name of R.G. Pearson created a means to calculate the absolute hardness by using the frontier orbitals. He determined that the absolute hardness equals the one half the difference between the ionization energy and the electron affinity. Notice that the equation below is very similar to the electronegativity equation for the Mulliken scale. The only difference is the subtraction.
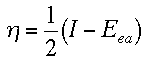 Hardness is dependent on the closeness of the frontier orbitals. When the HOMO and LUMO are close together, the absolute hardnes is low (because the energy difference between the two is very low) and the atom/molecule can easily share orbitals to create covalent bonds. When the frontier orbitals are far apart, their difference in energy is large. Typically, the ionization energy in the LUMO is high in this situation. With these atoms/molecules, the absolute hardess is high and they are not willing to share covalent bonds. Instead, they form ionic bonds. Some reference charts are given below for your convenience:
|
in cooperation with the
National Center for Supercomputing Applications
© Copyright 1999-2000 The Shodor Education Foundation, Inc.