ChemVizLab Activities |
Electronegativity: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization Energy
Students
TeachersReadings Lab Activities Support Materials Related Links Developers' Tools
|
Electronegativity is an atom's attraction for electrons in a bond. In
other words, it is a measure of the drawing power of an atom when it is part
of a compound. The greater the atom's power to attract electrons, the greater
the electronegativity. Electropositive is a term used to describe
elements with a very low electronegativity such as alkali metals. There are
three main scales use to calculate the electronegativity, the Pauling,
Mulliken, and Allred-Rochow scales. For our purposes, we will be talking
primarily about the Mulliken scale. The Mulliken scale calculates electronegativity as the average of the ionization energy and the electron affinity.
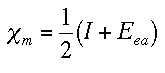 This scale is beneficial because it takes into account the differences in electronegativites of different orbitals as well as the atom's state of hybridization. Some electronegativity calculations are given below.
|
in cooperation with the
National Center for Supercomputing Applications
© Copyright 1999-2000 The Shodor Education Foundation, Inc.