HOME
Course Chapters
Calculator Fundamentals
Mathematics Review
Basic Concepts
Advanced Concepts
Section Tests
Pre-test
Post-test
Useful Materials
Glossary
Online Calculators
Redox Calculator
Kinetics Arrhenius Calculator
Thermodynamics Calculator
Nuclear Decay Calculator
Linear Least Squares Regression
Newton's Method Equation Solver
Compressibility Calculator
Units Conversion Calculator
Nomenclature Calculator
Related Information Links
Texas Instruments Calculators
Casio Calculators
Sharp Calculators
Hewlett Packard Calculators
Credits
Contact Webmaster
|
Chemical Nomenclature
Chemical nomenclature is the term given to the naming of compounds. Chemists use specific rules and "conventions" to name different compounds. This section is designed to help you review some of those rules and conventions.
Oxidation and Reduction
When forming compounds, it is important to know something about the way atoms will react with each other. One of the most important manners in which atoms and/or molecules react with each other is the oxidation/reduction reaction. Oxidation/Reduction reactions are the processes of losing and gaining electrons respectively. Just remember, "LEO the lion says GER:" Lose Electrons Oxidation, Gain Electrons Reduction. Oxidation numbers are assigned to atoms and compounds as a way to tell scientists where the electrons are in a reaction. It is often referred to as the "charge" on the atom or compound. The oxidation number is assigned according to a standard set of rules. They are as follows:
- An atom of a pure element has an oxidation number of zero.
- For single atoms in an ion, their oxidation number is equal to their charge.
- Fluorine is always -1 in compounds.
- Cl, Br, and I are always -1 in compounds except when they are combined with O or F.
- H is normally +1 and O is normally -2.
- The oxidation number of a compound is equal to the sum of the oxidation numbers for each atom in the compound.
Forming Ionic Compounds
Knowing the oxidation number of a compound is very important when discussing ionic compounds. Ionic compounds are combinations of positive and negative ions. They are generally formed when nonmetals and metals bond. To determine which substance is formed, we must use the charges of the ions involved. To make a neutral molecule, the positive charge of the cation (positively-charged ion) must equal the negative charge of the anion (negatively-charged ion). In order to create a neutral charged molecule, you must combine the atoms in certain proportions. Scientists use subscripts to identify how many of each atom makes up the molecule. For example, when combining magnesium and nitrogen we know that the magnesium ion has a "+2" charge and the nitrogen ion has a "-3" charge. To cancel these charges, we must have three magnesium atoms for every two nitrogen atoms:
3Mg2+ + 2N3- --> Mg3N2
Knowledge of the charges of ions is crucial to knowing the formulas of the compounds formed.
- alkalis (1st column elements) form "+1" ions such as Na+ and Li+
- alkaline earth metals (2nd column elements) form "2+" ions such as Mg2+ and Ba2+
- halogens (7th column elements) form "-1" ions such as Cl- and I-
Other common ions are listed in the table below:
| Positive ions (cations) | Negative ions (anions) |
|---|
| 1+ | 1- |
| ammonium (NH4+) | acetate
(C2H3O2-) |
| copper(I) (Cu+) | azide
(N3-) |
| hydrogen (H+) | chlorate
(ClO3-) |
| silver (Ag+) | cyanide (CN-) |
| | dihydrogen phosphate
(H2PO4-) |
| 2+ | hydride (H-) |
| cadmium (Cd2+) | bicarbonate
(HCO3-) |
| cobalt(II) (Co2+) | hydroxide
(OH-) |
| copper(II) (Cu2+) | nitrate
(NO3-) |
| iron (Fe2+) | nitrite
(NO2-) |
| lead (Pb2+) | perchlorate
(ClO4-) |
| manganese(II) (Mn2+) | permanganate
(MnO4-) |
| mercury(I)
(Hg22+) | thiocyanate(SCN-) |
| mercury(II) (Hg2+) | |
| nickel (Ni2+) | 2- |
| tin (Sn2+) | carbonate
(CO32-) |
| zinc (Zn2+) | chromate
(CrO42-) |
| | dichromate
(Cr2O72-) |
| 3+ | hydrogen phosphate
(HPO42-) |
| aluminum (Al3+) | oxide
(O2-) |
| chromium(III)
(Cr3+) | peroxide (O22-) |
| iron(III) (Fe3+) | sulfate
(SO42-) |
| | sulfide (S2-) |
| | sulfite (SO32-) |
| | 3- |
| | nitride (N3-) |
| | phosphate (PO43-) |
| | phosphide (P3-) |
Naming Ionic Compounds
The outline below provides the rules for naming ionic compounds:
Positive Ions
- Monatomic cations (a single atom with a positive charge) take the name of the element plus the word "ion"
Examples:
- Na+ = sodium ion
- Zn+2 = zinc ion
- If an element can form more than one (1) positive ion, the charge is indicated by the Roman numeral in parentheses followed by the word "ion"
Examples:
- Fe2+ = iron(II) ion
- Fe3+ = iron (III) ion
Negative Ions
- Monatomic anions (a single atom with a negative charge) change their
ending to "-ide"
Examples:
- O2- = oxide ion
- Cl- = chloride ion
- Oxoanions (negatively charged polyatomic ions which contain O) end in "-ate". However, if
there is more than one oxyanion for a specific element then the endings are:
| Two less oxygen than the most common starts with "hypo-" and ends with "-ite" |
One less oxygen than the most common ends with "-ite" |
THE MOST COMMON OXOANION ENDS WITH "-ATE" |
One more oxygen than the most common starts with "per-" and ends with "-ate" |
| ClO- = hypochlorite |
- ClO2- = chlorite
- NO2- = nitrite
- SO32- = sulfite
|
Most common oxyanions with four oxygens
- SO42- = sulfate
- PO43- = phosphate
- CrO42- = chromate
|
Most common oxyanions with three oxygens
- NO3- = nitrate
- ClO3- = chlorate
- CO32- = carbonate
|
|
ClO4- = perchlorate |
-
Polyatomic anions (a negatively charged ion containing more than one type of element) often add a hydrogen atom; in this case, the anion's name either adds "hydrogen-" or "bi-" to the beginning
Example:
CO32- becomes HCO3-
"Carbonate" becomes either "Hydrogen Carbonate" or "Bicarbonate"
-
When combining cations and anions into an ionic compound, you always put the cation name first and then the anion name (the molecular formulas are also written in this order as well.)
Examples:
- Na+ + Cl- --> NaCl
sodium + chloride --> sodium chloride
- Cu2+ + SO42- -->CuSO4
copper(II) + sulfate --> copper(II) sulfate
- Al3+ + 3NO3- --> Al(NO3)3
aluminum + nitrate --> aluminum nitrate
In naming ions, it is important to consider "isomers." Isomers are compounds with the same molecular formula, but different arrangements of atoms.
Thus, it is important to include some signal within the name of the ion that identifies which arrangement you are talking about. There are three
main types of classification, geometric, optical and structural isomers.
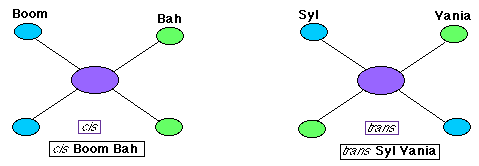
- Geometric isomers refers to which side of the ion atoms lie. The prefixes used to distinguish geometric isomers are cis meaning
substituents lie on the same side of the ion and trans meaning they lie on opposite sides. Below is a diagram to help you remember.
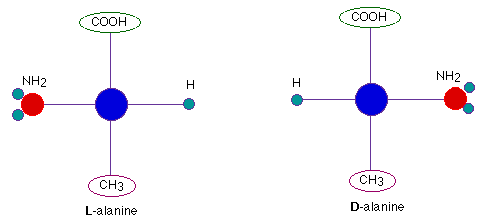
- Optical isomers differ in the arrangement of four groups around a chiral carbon. These two isomers are differentiated as L and D.
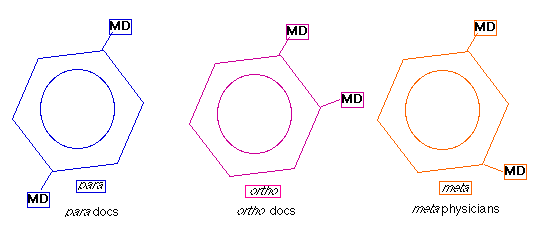
- Structural isomers differentiate between the placement of two chlorine atoms around a hexagonal carbon ring. These three isomers are identified as
o, m, and p. Once again we have given you a few clues to help your memory.
A pop-up nomenclature calculator is available for help when naming compounds and for practice problems.
Naming Binary Molecular Compounds
Molecular compounds are formed from the covalent bonding between non-metallic elements. The nomenclature for these compounds is described
in the following set of rules.
- The more positive atom is written first (the atom which is the furthest to the left and to the bottom of the periodic table)
- The more negative second atom has an "-ide" ending.
- Each prefix indicates the number of each atom present in the compound.
| Number of Atoms | Prefix | Number of
Atoms | Prefix |
|---|
| 1 |
mono |
6 |
hexa |
| 2 |
di |
7 |
hepta |
| 3 |
tri |
8 |
octa |
| 4 |
tetra |
9 |
nona |
| 5 |
penta |
10 |
deca |
Examples:
CO2 = carbon dioxide
P4S10 = tetraphosphorus decasulfide
Naming Inorganic Acids
- Binary acids (H plus a nonmetal element) are acids that dissociate into hydrogen atoms and anions in water. Acids that only release one hydrogen atom are known as monoprotic. Those acids that release more than one hydrogen atom are called polyproticacids. When naming these binary acids, you merely add "hydro-" (denoting the presence of a hydrogen atom) to the beginning and "-ic acid" to the end of the anion name.
Examples:
HCl = hydrochloric acid
HBr = hydrobromic acid
- Ternary acids (also called oxoacids, are formed by hydrogen plus another element plus oxygen) are based on the name of the anion.
In this case, the -ate, and -ite suffixes for the anion are replaced with -ic and -ous respectively. The new
anion name is then followed by the word "acid." The chart below depicts the changes in nomenclature.
| Anion name | Acid name |
| hypo___ite | hypo___ous acid |
| ___ite | ___ous acid |
| ___ate | ___ic acid |
| per___ate | per___ic acid |
Example:
ClO4- to HClO4 => perchlorate to perchloric acid
ClO- to HClO => hypochlorite to hypochlorous acid
Naming Compounds
A detailed treatise on naming organic compounds is beyond the scope of these materials, but some basics are presented. The wise chemistry student should
consider memorizing the prefixes of the first ten organic compounds:
| Number of Carbons
| Prefix
|
| 1 |
meth- |
| 2 |
eth- |
| 3 |
prop- |
| 4 |
but- |
| 5 |
pent- |
| 6 |
hex- |
| 7 |
hept- |
| 8 |
oct- |
| 9 |
non- |
| 10 |
dec- |
There are four basic types of organic hydrocarbons, those chemicals with only carbon and hydrogen:
- Single bonds (alkane): suffix is "ane", formula CnH2n+2
- Double bonds (alkene): suffix is "ene", formula CnH2n
- Triple bonds (alkyne): suffix is "yne", formula CnH2n-2
- Cyclic compounds: use prefix "cyclo"
So, for example, an organic compound with the formula "C6H14" would be recognized as an alkane with six carbons, so its name is "hexane".
Examples:
N2O4 = dinitrogen tetraoxide
S2F10 = disulfur decafluoride
Practice Problems
Find the formulas of the following molecules:
| 1. | aluminum fluoride | | | 8. | ammonium dichromate |
| 2. | carbon tetrachloride | | | 9. | magnesium acetate |
| 3. | strontium nitrate | | | 10. | zinc hydroxide |
| 4. | sodium bisulfate | | | 11. | nitric acid |
| 5. | iron(III) oxide | | | 12. | hypochlorous acid |
| 6. | mercury(II) nitrate | | | 13. | phosphoric acid |
| 7. | sodium sulfite | | | 14. | aluminum nitrate |
A solution set is available for viewing.
Write the names of the following molecules:
| 1. | CaCO3 | | | 8. | Mg3(PO4)2 |
| 2. | SCl2 | | | 9. | Ba(NO2)2 |
| 3. | Li2CrO4 | | | 10. | Hg2Cl2 |
| 4. | NaSCN | | | 11. | NaHCO3 |
| 5. | KClO3 | | | 12. | H2S |
| 6. | Ca(C2H3O2)2 | | | 13. | H2SO3 |
| 7. | K2Cr2O7 | | | 14. | SO3 |
A solution set is available for viewing.
[Basic Index]
[Chemical Nomenclature]
[Atomic Structure]
[Periodic Table]
[Lewis Structure]
[Chemical Reactions]
[Stoichiometry]
[Acid-Base Chemistry]
|

 Shodor
Shodor

