Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Chemical ThermodynamicsThe scientific discipline that intersects the areas of chemistry and physic is commonly known as physical chemistry, and it is in that area that a thorough study of thermodynamics takes place. Physics concerns itself heavily with the mechanics of events in nature. Certainly changes in energy -- however measured, whether it be heat, light, work, etc. -- are clearly physical events that also have a chemical nature to them. Thermodynamics is the study of energy changes accompanying physical and chemical changes. The term itself clearly suggests what is happening -- "thermo", from temperature, meaning energy, and "dynamics", which means the change over time. Thermodynamics can be roughly encapsulated with these topics:
Heat and WorkHeat and work are both forms of energy. They are also related forms, in that one can be transformed into the other. Heat energy (such as steam engines) can be used to do work (such as pushing a train down the track). Work can be transformed into heat, such as might be experienced by rubbing your hands together to warm them up.Work and heat can both be described using the same unit of measure. Sometimes the calorie is the unit of measure, and refers to the amount of heat required to raise one (1) gram of water one (1) degree Celsius. Heat energy is measured in kilocalories, or 1000 calories. Typically, we use the SI units of Joules (J) and kilojoules (kJ). One calorie of heat is equivalent to 4.187 J. You will also encounter the term specific heat, the heat required to raise one (1) gram of a material one (1) degree Celsius. Specific heat, given by the symbol "C", is generally defined as:
Where: C = specific heat in cal/g-°C
The value of C for water is 1.00 cal/g-°C. The values for specific heat that are reported in the literature are usually listed at a specific pressure and/or volume, and you need to pay attention to these settings when using values from textbooks in problems or computer models. Example Problem: If a 2.34 g substance at 22°C with a specific heat of 3.88 cal/g-°C is heated with 124 cal of energy, what is the new temperature of the substance?
Two other common heat variables are the heat of fusion and the heat of vaporization. Heat of fusion is the heat required to melt a substance at its melting temperature, while the heat of vaporization is the heat required to evaporate the substance at its boiling point. Chemical work is primarily related to that of expansion. In physics, work is defined as: Where: w = work, in joules (N×m) (or calories, but we are using primarily SI units) In chemical reactions, work is generally defined as :
The value of distance times area is actually the volume. If we imagine a reaction taking place in a container of some volume, we measure work by pressure times the change in volume. Where: ΔV is the change in volume, in liters If ΔV=0, then no work is done. Example Problem: Calculate the work that must be done at standard temperature and pressure (STP is 0°C and 1 atm) to make room for the products of the octane combustion:
EnergyYou might remember the first law of thermodynamics: energy cannot be created or destroyed. Energy can only change form. Chemically, that usually means energy is converted to work, energy in the form of heat moves from one place to another, or energy is stored up in the constituent chemicals. You have seen how to calculate work. Heat is defined as that energy that is transferred as a result of a temperature difference between a system and its surroundings. Mathematically, we can look at the change in energy of a system as being a function of both heat and work:
Where: ΔE is the change in internal energy of a system
If q is positive, we say that the reaction is endothermic, that is, heat flows into the reaction from the outside surroundings. If q is negative, then the reaction is exothermic, that is, heat is given off to the external surroundings. You might also remember the terms kinetic energy and potential energy. Kinetic energy is the energy of motion -- the amount of energy in an object that is moving. Potential energy is stationary, stored energy. If you think of a ball sitting on the edge of a table, it has potential energy in the energy possible if it falls off the table. Potential energy can be transformed into kinetic energy if and when the ball actually rolls off the table and is in motion. The total energy of the system is defined as the sum of kinetic and potential energies. In descriptions of the energy of a system, you will also see the phrase "state properties". A state property is a quantity whose value is independent of the past history of the substance. Typical state properties are altitude, pressure, volume, temperature, and internal energy.
Where: ΔH = change in enthalpy We define enthalpy itself as:
Where:
H = enthalpy You will not need to be able to calculate the enthalpy directly; in chemistry, we are only interested in the change in enthalpy, or ΔH.
Example Problem: Calculate the ΔH value of the reaction:
We can also represent enthalpy change with the equation:
Where:
ΔV is the change in volume, in liters If you recall, work is defined as P ΔV, so enthalpy changes are simply a reflection of the amount of energy change (energy going in or out, endothermic or exothermic), and the amount of work being done by the reaction. For example, if ΔE = -100 kJ in a certain combustion reaction, but 10 kJ of work needs to be done to make room for the products, the change in enthalpy is:
This is an exothermic reaction (which is expected with combustion), and 90 kJ of energy is released to the environment. Basically, you get warmer. Notice the convention used here -- a negative value represents energy coming out of the system. You can also determine ΔH for a reaction based on bond dissociation energies. Breaking bonds requires energy while forming bonds releases energy. In a given equation, you must determine what kinds of bonds are broken and what kind of bonds are formed. Use this information to calculate the amount of energy used to break bonds and the amount used to form bonds. If you subtract the amount to break bonds from the amount to form bonds, you will have the ΔH for the reaction.
Example Problem: Calculate ΔH for the reaction:
EntropyEntropy is a measure of the disorder of a system. Take your room as an example. Left to itself, your room will increase in entropy (i.e., get messier) if no work (cleaning up) is done to contain the disorder. Work must be done to keep the entropy of the system low. Entropy comes from the second law of thermodynamics, which states that all systems tend to reach a state of equilibrium. The significance of entropy is that when a spontaneous change occurs in a system, it will always be found that if the total entropy change for everything involved is calculated, a positive value will be obtained. Simply, all spontaneous changes in an isolated chemical system occur with an increase in entropy. Entropy, like temperature, pressure, and enthalpy, is also a state property and is represented in the literature by the symbol "S". Like enthalpy, you can calculate the change of S (ΔS).
Where:
ΔS is change in entropy The following table shows the relationship between the state of a substance and its entropy:
Where:
G is the energy (sometimes called the free energy) You can also calculate the change in G the same way as you calculate the change in enthalpy or entropy:
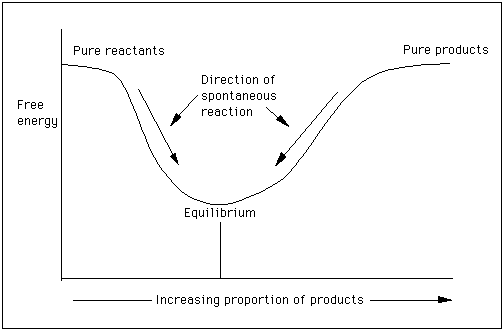
Where: ΔG is change in free energy A pop-up calculator is available to calculate the enthalpy and Gibbs free energy changes in reactions. Given a constant temperature and pressure, the direction of any spontaneous change is toward a lower Gibbs free energy. The graphic below shows that during a reaction, the amount of free energy decreases until the reaction is at equilibrium. If the reaction goes towards completion, the free energy minimum occurs very close to the pure products part of the curve. In other words, the curve moves depending on the conditions of the reaction.
 A table relating all of the state properties summarized above -- enthalpy change, entropy change, and change in free energy -- is shown below. A spontaneous reaction is one that occurs without any outside intervention. Processes that are spontaneous in one direction are non-spontaneous in the reverse direction.
Enthalpy Practice Problem: Given the following bond dissociation energies (H-C is 413 kJ/mol; H-H is 436 kJ/mol; C=C is 614 kJ/mol; C-C is 348 kJ/mol), determine ΔH for the reaction:
Entropy Practice Problem: Given the following entropy values Al2O3(s) is 51.00 kJ/mol; Al(s) is 28.32 kJ/mol; H2O(g) is 188.7 kJ/mol; H2(g) is 130.6 kJ/mol, determine ΔS for the reaction:
[Advanced Index]
[Gas Laws]
[Thermodynamics]
[Kinetics]
[Equilibria] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
WebMaster@shodor.org