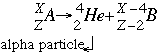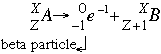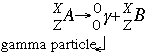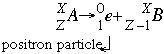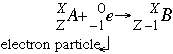
HOME
Course Chapters
Calculator Fundamentals
Mathematics Review
Basic Concepts
Advanced Concepts
Section Tests
Pre-test
Post-test
Useful Materials
Glossary
Online Calculators
Redox Calculator
Kinetics Arrhenius Calculator
Thermodynamics Calculator
Nuclear Decay Calculator
Linear Least Squares Regression
Newton's Method Equation Solver
Compressibility Calculator
Units Conversion Calculator
Nomenclature Calculator
Related Information Links
Texas Instruments Calculators
Casio Calculators
Sharp Calculators
Hewlett Packard Calculators
Credits
Contact Webmaster
|
Nuclear Chemistry
Nuclear chemistry deals with the nuclei of atoms breaking apart. Atoms are continually undergoing decay. When studying nuclear chemistry, there is a typical format used to represent specific isotopes.
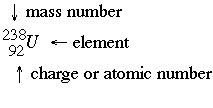
Nuclear equations are typically written in the format shown below. There are 5 different types of radioactive decay.
- Alpha decay follows the form:
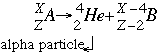
Where A is the parent isotope (the atom being broken apart) B is the daughter isotope or the isotope formed. When an element is broken down in alpha decay it looses two neutrons and two (2) protons. This means that the name of the element will change as well, moving back two (2) places on the periodic table. Alpha decay is not very penetrating because the He atoms capture electrons before traveling very far. However it is very damaging because the alpha particles can knock atoms off of molecules. Alpha decay is the most common in elements with an atomic number greater than 83.
- Beta negative decay follows the form:
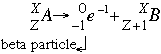
The beta emission increases the atomic number by one (1) by adding one (1) proton. At the same time, one (1) neutron is lost so the mass of the daughter isotope is the same as the parent isotope. Beta negative decay is more penetrating than alpha decay because the particles are smaller, but less penetrating than gamma decay. Beta electrons can penetrate through about one (1) cm of flesh before they are brought to a halt because of electrostatic forces. Beta decay is most common in elements with a high neutron to proton ratio.
- Gamma decay follows the form:
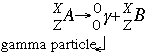
In gamma emission, neither the atomic number or the mass number is changed. A high energy gamma ray is given off when the parent isotope falls into a lower energy state. Gamma radiation is the most penetrating of all. These photons can pass through the body and cause damage by ionizing all the molecules in their way.
- Positron emission (also called Beta positive decay) follows the form:
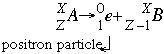
In this reaction a positron is emitted. A positron is exactly like an electron in mass and charge force except with a positive charge. It is formed when a proton breaks into a neutron with mass and neutral charge and this positron with no mass and the positive charge. Positron emission is most common in lighter elements with a low neutron to proton ratio.
- Electron capture follows the form:
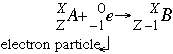
In this reaction a nucleus captures one (1) of its own atom's inner shell electrons which reduces the atomic number by one. This captured electron joins with a proton in the nucleus to form a neutron. Electron capture is common in larger elements with a low neutron to proton ratio.
All elements with an atomic number over 83 are considered radioactive. Radioactivity can be measured using a geiger counter, a cylinder containing a low-pressure gas and two (2) electrodes. Radiation ionizes the atoms in the cylinder and allows current to flow between the electrodes.
All radioactive elements disintegrate according to their specific half life. The half life of a radioactive substance is the time required for half of the initial number of nuclei to disintegrate. The decay rate expresses the speed at which a substance disintegrates. The following equation represents the relationship between the number of nuclei remaining, N, the number of nuclei initially present, N0, the rate of decay, k, and the amount of time, t.
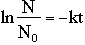
The relationship between the half-life of a radioactive substance and k, the rate at which it decays can also be found.
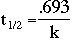
By using these equations, it is possible to calculate how much of a nuclear substance will be left after a certain time and how much of a substance originally existed. A common example is isotopic dating in which the ages of archeological artifacts are determined by measuring the activity of the isotopes.
Practice Problems
1. The half life of a specific element was calculated
to be 5200 yr. Calculate the decay constant (k). Solution.
2. If a watch contains a radioactive substance with a
decay rate of 1.40*10-2 and after 50 years only 25 mg remain, calculate the amount originally present. Solution.
3. A rock contains 0.257 mg of lead-206 for every mg of
uranium-238. The half-life decay for uranium to turn into lead is 4.5x109 yr. How old is the rock? Solution.
A Nuclear Calculator and a
Disintegration Time Calculator are available for use.
[Advanced Index]
[Gas Laws]
[Thermodynamics]
[Kinetics]
[Equilibria]
[Redox Reactions]
[Nuclear Chemistry]
|

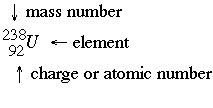
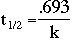
 Shodor
Shodor