Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Problem 4 SolutionSolution Steps:Don't let the technical terms get in the way! We are given a ratio:
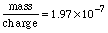 Substituting in gives: 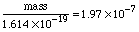 To finish this problem, we need to multiply these scientific notation numbers. Using the EE key: The calculator prints: So we'll state the final answer as: Why only 3 digits? Look at the number of significant digits in the given measurements. The smallest is 3, so we can hope for no more than 3 digits in the answer. Why put kilograms with this answer? In the last two problems, we were looking at whole atoms. Here we are looking at mass and charge, which are measurements and have units associated with them. Charge was given coulombs and the mass to charge ratio was given in kilograms per coulomb, and so we know mass : charge must have corresponding units kilogram : coulomb. In the next topic we review units and dimensions and address these issues. |
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
WebMaster@shodor.org