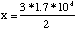
Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Another Problem 4Question:In the synthesis of ammonia from nitrogen and hydrogen, 3 moles of hydrogen are needed for every 2 moles of ammonia produced. If we want to make 1.7x104 moles of ammonia, how much hydrogen do we need?
Solution: 25500 moles.
|
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
WebMaster@shodor.org