ChemVizLab Activities |
Background Reading for Z-Matrices: |
|
Z-Matrices
Students
TeachersCartesian Converter Materials Readings Lab Activities Support Materials Related Links Developers' Tools
|
Key PointsBy now you should be familiar with the diagram below. 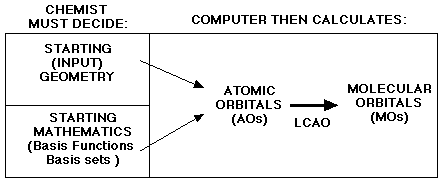 As you may recall, there are two main decisions that you, the scientist, must make before submitting input files to computational chemistry programs. You must decide on the geometry of the molecule and on a starting set of mathematics and approximations known as the basis sets. Today we are going to deal with the first, the molecular geometry. Every molecule has multiple geometries. Choosing the correct molecular equilibrium geometry is very important when carrying out computational studies. This is because the energy of a molecule depends on its geometry. Even small changes in the structure can lead to drastic changes in total energy. The geometry of a molecule can be described using one of three different methods. The first is by using cartesian coordinates. Using the x-y-z coordinate system, the scientist must identify the coordinates for each atom in the molecule. However, this method is only efficient for small molecules. 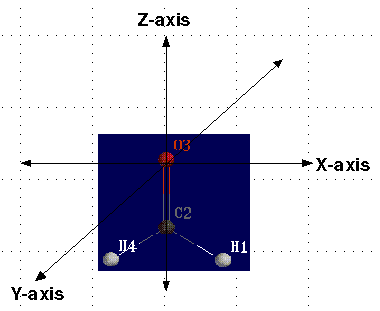  The second method uses a molecular editor or graphical user interface (GUI). These are computer programs which allow you to construct various molecules. The program then automatically calculates the geometry of the molecule. GUIs work well for larger molecules. The third method is called a Z-Matrix. The Z-Matrix is a simple, but rough, geometrical approximation. It works by identifying each atom in a molecule by a bond distance, bond angle and dihedral angle in relation to other atoms in the molecule. Z-Matrices work well for large molecules because the Z-Matrix can be easily converted to cartesian coordinates using Shodor's Z-Matrix Conversion Tool. A dihedral angle is formed from four atoms, and helps to define the dimensionality of the molecule.
Building a Z-Matrix:When constructing a Z-matrix, you should follow these steps:
Practice Examples:Water MoleculeConverting Z-Matrices to Cartesian Coordinates:As we mentioned above, a z-matrix is used as input for computer programs which convert the information into cartesian coordinates. You will need to understand how to input the z-matrix into one of these programs in order to get your cartesian coordinates for other calculations. We will be using a program created for ChemViz users. The program can be found on the internet at http://www.shodor.org/chemviz/babel/html. |
in cooperation with the
National Center for Supercomputing Applications
© Copyright 1999-2000 The Shodor Education Foundation, Inc.