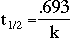
HOME
Course Chapters
Calculator Fundamentals
Mathematics Review
Basic Concepts
Advanced Concepts
Section Tests
Pre-test
Post-test
Useful Materials
Glossary
Online Calculators
Redox Calculator
Kinetics Arrhenius Calculator
Thermodynamics Calculator
Nuclear Decay Calculator
Linear Least Squares Regression
Newton's Method Equation Solver
Compressibility Calculator
Units Conversion Calculator
Nomenclature Calculator
Related Information Links
Texas Instruments Calculators
Casio Calculators
Sharp Calculators
Hewlett Packard Calculators
Credits
Contact Webmaster
|
Chemistry Fundamentals Program |
Nuclear Decay Calculator
Use this calculator to investigate how a unstable substance decays over
time. The first two equations are found in the Nuclear Chemistry section.
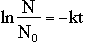
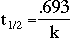
From the above two equations, we derive the following, which we use as the mathematical basis
for calculating decay.
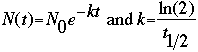 Here, t1/2 is the half-life of the element, which is specific to each
element. By selecting an element from the table below, you are specifying the half
life that appears in the table. You also must enter an initial number of moles of nuclei, and the
amount of time that you would like to consider. Note that you must also select the appropriate
units of time.
Here, t1/2 is the half-life of the element, which is specific to each
element. By selecting an element from the table below, you are specifying the half
life that appears in the table. You also must enter an initial number of moles of nuclei, and the
amount of time that you would like to consider. Note that you must also select the appropriate
units of time.
| Element |
Half-life |
|
Element |
Half-life |
| Uranium-238 |
4.4x109 years |
Carbon-14 |
5.73x103 years |
| Potassium-40 |
1.3x109 years |
Phosphorus-32 |
14.28 days |
| Uranium-235 |
7.1x108 years |
Magnesium-27 |
9.46 minutes |
| Iodine-129 |
1.7x107 years |
Magnesium-20 |
0.6 seconds |
Any scientific notation should be entered in the form (XeY) where "eY" represents multiplying by 10Y
(e.g. 1.09*105 equals 1.09e5)
|

 The Shodor Education Foundation, Inc.
The Shodor Education Foundation, Inc.