

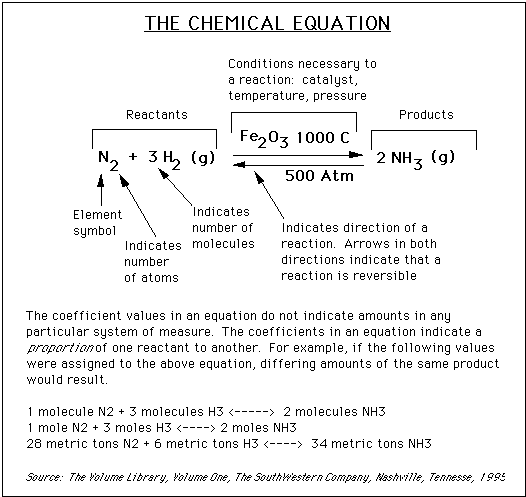
The first calculation is quite simple: the gram molecular weight, or GMW. Each element has a certain mass, usually measured in units of grams. Nitrogen (N2) has a mass of 14 grams, so the molecular weight is 2 x 14 grams, or 28 grams. Similarly, the mass of the hydrogen molecule is 2 x 1.0 grams, or 2.0 grams, and the mass of the NH3 is 14.0 grams + (3 x 1.0) or 17 grams.
Probably the most common unit of measurement in chemistry is the "mole", which might be considered to be an abbreviation for gram MOLEcular weight. One mole of anything is its gram molecular weight. For example, one mole of N2 is 28 grams. One mole of H2 is 2.0 grams. One mole of NH3 is 17 grams. It is critical, if you are to survive in chemistry, that you be able to quickly do mass-to-mole conversions and mole-to-mass conversions:
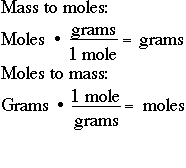
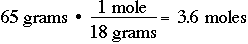
Likewise, you should be able to convert moles to grams. If you have 28.6 moles of carbon dioxide, CO2, calculate the number of moles of CO2: CO2 has a mass of 12 + (2 X 16) grams = 44 grams:
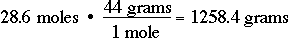
One other useful calculation in stoichiometry (there will be more!) is determining the number of atoms in some amount of a chemical. For example, if I have 35 grams of water, how many individual water atoms do I have?
The conversion factor is a number known as "Avogadro's number" (one of those that some of us older chemists think students should memorize, but we'll let your professors decide that for you!). Avogadro's number is 6.023 x 1023 atoms per mole, or:
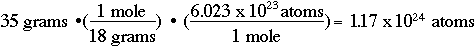
Special Challenge Problem!
Density is a common measurement in chemistry, and refers to the amount of a substance (measured in grams) in a specific volume of a liquid (measure in milliliters, mL, or cubic centimeters, cm3). Mercury has a density of 13.534 g/cm3. If you have 62.5 cm3 of mercury, how many:
Solution:
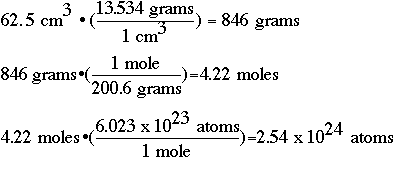
Keep in mind that attention needs to be paid to significant figures! Most of these calculations involve multiple steps, and it would be well worth your time to SETUP the problems as we have done here. You should notice that if you do the set-up correctly, the units will cancel out, and you will be left with the unit that you are trying to find. This should give you a "warm fuzzy" that you have multiplied or divided appropriately.