

There are a variety of units for concentrations of solutions. The most common is molarity. Molarity is defined as the number of moles of solute (usually solid stuff) that has been dissolved in one liter (1000 milliliters or 1000 cm3). A 1.50-M (read "M" as "molar") solution would have 1.5 moles of solid solute dissolved in one liter of liquid (usually water).
Several examples should make this session clear. Suppose we wish to prepare a 3.0 molar solution of NaCl (table salt). The first step is to calculate the number of grams in 3.0 moles of NaCl. From the reading on stoichiometry, you should be able to do this calculation:

To make a 3.0 M solution, you simply dissolve 175.5 grams in one liter (1000 milliliters) of water, and you have what you need.
More typically, you will need to prepare a specific amount of a solution of a given molarity. For example, suppose you need 50.0 mL of a 0.02 M solution of sodium hydroxide, NaOH. How many grams of NaOH must be dissolved in 50.0 mL of water?
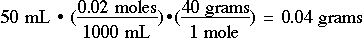
The point we are trying to make with these exercises is that the successful chemistry student must be able to not only understand the chemical concepts, but must be able to accurately and readily perform multi-step calculations. As a reminder, the purpose of these sessions is to not only help you to use your calculator, but also to help you develop an awareness of the kinds of calculations you will be expected to do early on in your chemistry studies!