

The generic formula for any pX calculation is:
pX = -log[X] where [X] is the concentration of X, normally in units of moles/liter (same as molarity).
Before we get to the business of calculating pX values on the calculator, you should be able to "eyeball" concentrations and estimate their pX values. This is not only helpful when you just need a "quick and dirty" idea of the pX, but also serves as a good check that you have pushed the correct buttons on your calculator!
You should recall that a logarithm is defined as:
For example, log (105) = 5
You might also recall that numbers greater than 1 (such as 100, 000, or 105) have a positive log value, while numbers smaller than 1 (such as 0.001, or 10-3, have a negative log value).
Here is a sample pH calculation. The concentration of the hydrogen ion (H+) in solution is 1.0 x 10-5. What is the pH of the solution? If you look at the value of the exponent, in this case the value "-5", you should know that the log of 1.0 x 10-5 is -5. Since by definition, pH = -log[H], once you figure out the log of 1.0 x 10-5, you simply remove the minus sign (i.e., a minus times a minus is a positive). The pH of this solution is therefore 5.
Calculating pH values for which the number in front of the "x 10-5" is 1.0 is easy. However, suppose that there is some other number greater than 1.0, such as [H] = 3.0 x 10-5? How do you "eyeball" these concentrations? First, evaluate the pH as if the number were 1.0. You recognize that this pH is 5.0. Since 3.0 is greater than 1, the concentration is moving towards 10 x 10-5 , which is the same as 1.0 x 10-4. So the true pH is somewhere between 5 and 4. Where exactly? Being able to predict the true value requires a little practice and experience, but we might think this way: well, 3 is less than halfway between 1 and 10, so the pH is probably between 4.5 and 5. If on your calculator (or on a multiple choice test!), a pH value is presented that is LESS THAN 4.5, you might be suspicious! The calculator suggests that the true value is 4.52. The chart and graphic below show how pH changes as you move from a small value (1.0 x 10-5) to a larger value (1.0 x 10-4):
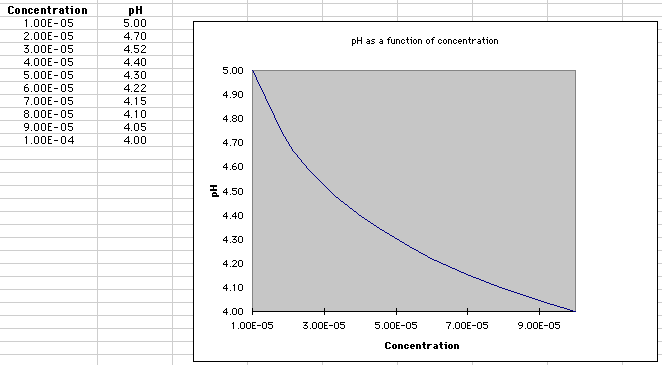
You should notice that the decrease in pH from 1.0 x 10-5 to 1.0 x 10-4 does not happen in a straight line, but exponentially. As such, being able to guess an approximate value for pH comes with experience and more familiarity with exponential curves!
To calculate pH on any calculator is relatively straightforward:
Calculate the pH of a solution that has the following concentration of H+ ions: 4.6 x 10-3
You've already had the discussion on significant figures, so report the pH as appropriate!
You should also be able to calculate the concentration of X, given pX:
What is the concentration of a solution that has a pH of 6.8?
You should be able to apply the "eyeball" test to this result. Based on the "-7" exponent, you should have a good feeling that the pH is around 7. Since 1.58 is more than 1, the actual pH should be something less than 7, in this case 6.8!