Dissolved Oxygen Activity
Lesson - Dissolved OxygenStream Assimilation of Organic Wastes and the Impacts on Dissolved OxygenSteven I. GordonThe Ohio Supercomputer CenterJune 2005Introduction One of the most important measures of the health of the stream is the level of dissolved oxygen (DO) in the water. Oxygen (O2) dissolves in water through the mixing of the water surface with the atmosphere. The oxygen is used by fish and other animals in the water to "breath" through their gills or other respiratory systems and by plants. If the levels fall too low, many species of fish, macroinvertebrates, and plants cannot survive. At very low levels of oxygen, the stream becomes "septic" and smells rotten because low oxygen sulfur bacteria begin to dominate. The level of oxygen dissolved in the water is inversely related to the water temperature. The lower the temperature, the more oxygen can dissolve in the water. At zero degrees centigrade, the maximum or saturation level of DO is 14.6 parts per million (ppm) or for every million molecules of water, there are 14.6 molecules of oxygen. Because of the molecular weight of water, ppm of dissolved substances in water is also equivalent to milligrams of the substance per liter of water. As water becomes warmer, the saturation amount of DO will drop. For example, at 30 degrees centigrade, the DO saturation is 7.56 mg/l while at 40 degrees it goes down to 6.41 mg/l. Even in very clean streams, the DO never quite gets to the saturation DO level as animals and plants consume oxygen for respiration and bacteria decompose natural wastes, also using oxygen for respiration. When people discharge sewage or animal wastes into streams, those wastes are decomposed by bacteria. Because the discharge is a very large amount coming out of a pipe at one location, there is an immediate depression in oxygen levels from the mixing of the sewage volume with low oxygen levels with the stream water with higher oxygen levels: Average DO = (DO_effluent*Sewage_Flow_+DO_stream*Stream_Flow)/
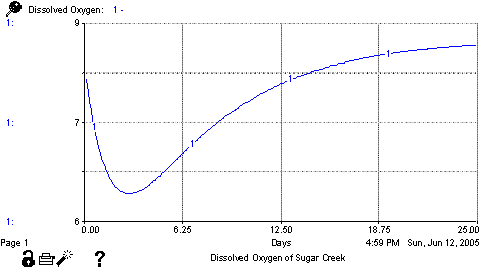
Once discharge occurs, bacteria begin to decompose the wastes, using oxygen from the water in the process. The amount of oxygen that might be used is measured by a test called the Biochemical Oxygen Demand (BOD). For each increment of time, a portion of the BOD is decomposed based on a decay rate for decomposition and as a result, an increment of oxygen is consumed. At the same time, additional oxygen is dissolved in the water in exchange with the atmosphere. The rate at which this reaeration happens is governed by the characteristics of the stream and is often expressed as a function of the stream depth and velocity. Taken together this deoxygenation and reaeration produce an oxygen sag curve that looks like figure 1.
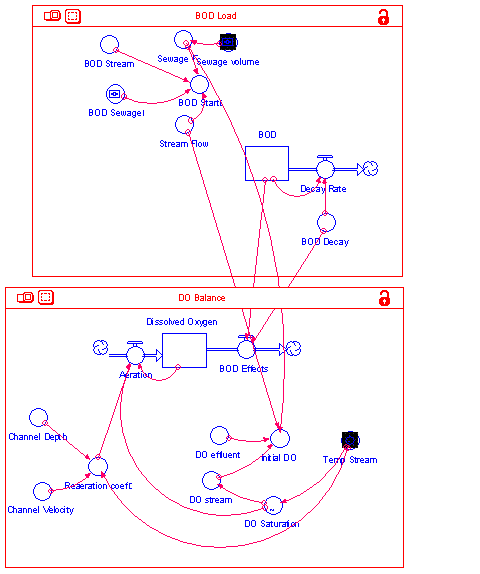
The oxygen level continues to drop as the amount of oxygen consumed exceeds the rate of reaeration. Once enough of the BOD load has been consumed, the trend reverses as the rate of reaeration exceeds the rate of deoxygenation and the oxygen level continues to rise until it approaches the saturation level. This system was modeled originally by Streeter and Phelps in 1925 and has become the basis for modeling the impacts of sewage treatment and industrial plants on oxygen levels in streams. In reality, the system is much more complicated as plants add oxygen to the water during photosynthesis and other biochemical processes consume oxygen at different rates. However, it is still a realistic approximation of the trends that can be used to test the impacts of sewage treatment plants on the receiving stream. Using Stella, we have created a version of the model which represents the dissolved oxygen of Sugar Creek in Stark County, Ohio. The model is based on a much more complete and complex model of the system developed for USEPA (Brown and Barnwell, 1987). The Stella model is divided into two major sectors. The BOD Load sector calculates the initial organic waste load and its decay with time. The DO Balance sector calculates the original DO level as a function of temperature and the mixing of the waste load and then tracks the impacts of BOD decay on oxygen levels over time. The model is shown in figure 2.
Figure 2: DO Model Diagram The BOD sector starts with the calculation of BOD loads. The current sewage volume from the sewage treatment plant is 1.03 cubic feet per second. This is converted into liters per day to make it compatible with the other calculations. The resulting volume is then assumed to mix with the stream. The concentrations of BOD in the sewage (33 ppm) and river (5.95 ppm) are multiplied by the respective flow volumes to attain the starting average BOD load at the place where the sewage treatment plant discharges. This becomes the initial level of BOD to be degraded by bacteria in the stream. The decay occurs with each increment of time by a constant decay rate. The DO calculations start with the lookup of the DO saturation level based on the current stream temperature. There is then a similar calculation of the average DO level based on the mixing of the effluent with the stream water. For each time increment, the DO declines as a result of BOD causing deoxygenation and it increases via reaeration. This continues with time until the BOD load is decomposed and the oxygen level increases toward the saturation level. In the stream, this actually happens downstream of the original discharge as the stream flows away from the discharge point. The results are shown in several graphs and tables that track the levels of BOD and DO. There are slider bars that allow adjustments to the sewage load which related to the volume of discharge, the concentration of the BOD in the sewage which relates to the efficiency of treatment at the sewage treatment plant, and to the temperature to reflect conditions at different times of year. The assumptions of the model are: There is only one point source of pollution and no stormwater or non-point sources of pollution. There is complete mixing of the effluent with the stream that occurs immediately and is constant downstream. The stream does not significantly change in volume, velocity, or depth as we go downstream from the discharge so that the reaeration and deoxygenation rates are constant. The temperature and pressure are constant. There are no significant sources of oxygen from plants, oxygen use by animals, or oxygen use to decompose wastes in the sediment on the stream bottom. Testing Different Sewage Treatment Options Run the model in its initial form and note the impacts of the sewage treatment plant on the DO. What is the minimum DO and when is it reached? Compare the DO level with the information on DO requirements for aquatic species found in the Water on the Web page referred to below. Rerun the model assuming the worst summer conditions where the temperatures are expected to be 75 degrees Fahrenheit. Describe the impacts of the warmer temperatures. The local watershed protection association is in a battle with the municipal authorities over plant upgrades and expansions. The operators of the plant want to improve the plant's performance as well as increase its capacity in response to anticipated growth. The current plant does not meet the EPA discharge requirements of 25 ppm. In addition, they wish to expand its capacity to nearly double current capacity to 2.0 cfs. Use the model to test the impacts of an upgrade with expansion, an upgrade with expansion, and the impacts of summer high temperatures on the stream. The watershed association has suggested building a plant which exceeds standards and treats the waste to a concentration of 15 ppm. What should be done and how will it impact the assimilative capacity of the stream? |