One of the main concerns of atmospheric chemists is: how do pollutants emitted into the atmosphere in one country or hemisphere flow into other countries or parts of the globe? Recently there have been significant political and diplomatic difficulties between the United States and Canada about this issue. Since pollutants know no boundaries,
many of the pollutants that cause acid rain (mostly sulfur dioxide) are transported by winds in the upper atmosphere into Canada, where they mix with rain to produce acid rain.
In this case study, we are concerned with the flow of a particular pollutant, ethane, between the Northern and Southern hemispheres. Ethane (C2H6) is a part of natural gas that is emitted to the atmosphere whenever natural gas escapes unburned at wells and other sources. Ethane only enters the atmosphere in this fashion. In the atmosphere, ethane undergoes a number of chemical transformations (mostly oxidation with the hydroxyl radical, OH ) to form peroxyacetyl nitrate, also known as PAN. PAN is a part of the smog and ozone problems, and causes severe eye irritations and respiratory problems, particularly among the elderly and those with respiratory diseases. ) to form peroxyacetyl nitrate, also known as PAN. PAN is a part of the smog and ozone problems, and causes severe eye irritations and respiratory problems, particularly among the elderly and those with respiratory diseases.
In the Northern Hemisphere, ethane has a concentration of about 1.0 parts per billion (ppb). With a concentration of 1 ppb, that means there is one molecule of ethane for a billion molecules of air molecules. Using a golf analogy, if the concentration of red golf balls was 1 ppb, there would be one red golf ball for every 1,000,000,000
white golf balls. This may not seem like a problem, but this one little molecule of ethane reacts chemically with other stuff in the atmosphere, and eventually becomes a problem. It is like rolling a small snowball off a long hill - a little snowball won't hurt anyone, but if it rolls long enough, it becomes a snow boulder that can do serious damage!
In the Southern Hemisphere, the typical concentration of ethane is 0.5 ppb. Ethane can exit from the atmosphere by any of three methods, or mechanisms:
- it can flow out of the lower atmosphere (the troposphere) where it does the most harm to the upper atmosphere (the stratosphere) where it does little or no harm.
- it can wash out of the atmosphere through mixing with rain or snow.
- it can react with other chemicals and be transformed to something different.
Ethane can also flow between hemispheres. Ethane can flow from North to South and South to North. The graphic below shows all of the possible mechanisms:
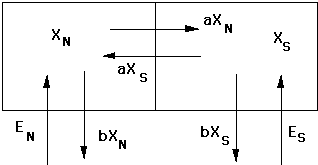
In this graphic, we show the Northern Hemisphere (XN) on the left and the Southern Hemisphere (XS) on the right. We show ethane coming into the two hemispheres from the bottom (EN and ES) . The graphic also shows ethane moving between hemispheres (aXN and aXN). In this case, the letter "a" (alpha) is a rate that shows the number of ethane molecules transported per some period of time. For example, if alpha is 80%/year, then 80% of the ethane in the Northern Hemisphere and 80% of the ethane in the Southern Hemisphere change places every year. If the amounts in both are the same, then the net difference is zero -- this is called the steady-state or equilibrium condition. The politicians would love this to be true -- the stuff that is emitted in the North stays in the North, and the stuff emitted in the South stays in the South!
The graphic also shows ethane leaving both the North and the South through "wet deposition" -- pollutant mixes with the rain and snow and is deposited on the ground, where it may or may not do damage to people and things. Acid rain is the classic example of "wet deposition" - the stuff deposited is acid rain, which destroys buildings, plants, farm crops, and kills living things in ponds and lakes. The letter "b" (beta) is the rate constant for wet deposition from the atmosphere to the ground.
|