Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Problem 4 SolutionA sample thought to be caffeine is tested and the resulting composition was 53% carbon, 4% hydroge, 30% nitrogen and 13% oxygen. We know from theory that one mole of caffeine contains 96.08 g carbon, 10.08 g hydrogen, 56.04 g nitrogen and 32.00 g oxygen. Was the sample really caffeine? No, it was not caffeine. Solution Steps:We need to compare the percent composition of the unknown sample to the known amounts in caffeine. We can't compare amounts with percents so we need to change the amounts into percents. First we'll find the total number of grams in one mole of caffeine:
96.08 + 10.08 + 56.04 + 32.00 = 194.20 Now we can figure the percent of each element in caffeine:
hydrogen: 10.08 / 194.20 = .051905252 = 5.19%
nitrogen: 56.04 / 194.20 = .288568486 = 28.86% oxygen: 32.00 / 194.20 = .164778579 = 16.48% Are the percents from the sample close to the percents from caffeine? Not really. How far off are they? Here's a good place to check % error! Let's look at the % error in the oxygen figure:
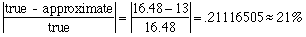 That's off by more than 20%! Not likely! Another Problem Like This One.
|
 The Shodor Education Foundation, Inc.
The Shodor Education Foundation, Inc.in cooperation with the Department of Chemistry,
Appalachian State University
Copyright © 1998
Last Update:
Please direct questions and comments about this page to
WebMaster@shodor.org