Course Chapters
Section Tests
Calculators Linear Least Squares Regression Newton's Method Equation Solver
|
Units, Dimensions, and ConversionsWhen measuring a quantity, the units we use are just as important as the numerical value we obtain. Stating that the mass of an object is one (1) says very little about the actual mass of the object. It is perfectly reasonable for an instructor to respond to such an answer: "One what???"
So, what are the basic units of measurement? Let's start with a list of some things we might measure:
Each of these quantities has a variety of units we might use. Here are a few examples.
The units in bold font are the basic units used in the SI System of Units the recommended scientific system of units. In addition to length, mass, time and temperature, the list of basic quantities includes three others you may not have seen before: electric current (ampere), luminous intensity (candela) and amount of a chemical substance (mole). The last unit (mole) is constantly used in chemistry and explained on the stoichiometry page. So, what do you need to know?
Combinations of UnitsWhen studying chemistry you will measure many quantities whose units are actually combinations of the basic units. Be sure to include the units in your notes when you encounter a new term. Here is a list of quantities that you should already be familiar with from previous classes. Follow the link to a short description for those you don't recall.
Unit ConversionsChanging between units is easy if we have a conversion equation. For example, Robert Millikan (a 20th century physicist) performed a landmark experiment with x-rays and determined the mass of an electron to be 9.1x10-31 kg. While this is the standard way to represent this quantity, it would also be correct to use grams (g) or milligrams (mg):
How do we change between units like this? Here are two conversion equations that will help in this situation:
Notice that we can rearrange these equations in several ways dividing by one of the sides:
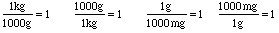 We have formed several ratios which are all equal to 1! Now we need to remember a crucial fact from arithmetic:
This is the key to changing units! Consider changing from kg to g. Which ratio equal to 1 should we multiply kilograms by to get grams? Well, if we use the fraction with grams on top and kilograms on bottom, kilograms will "cancel out" when we simplify. Watch:
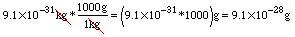 Notice that we multiplied numbers in scientific notation. We can use the EE button on a calculator as follows:
The calculator prints: If you are still unsure about how to handle scientific notation on your calculator see the section on Calculator Fundamentals. Changing from kilograms to milligrams works in the same way, except we need to convert from kg to g and then from g to mg since we aren't given a single kg-to-mg conversion equation. We can combine these into one step, though! Watch:
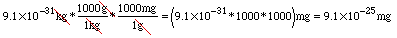 We just multiplied by "one" twice, choosing the ratios that allowed us to cancel out the kg and the g units. So what other conversion equations are there? Click here to look at the ones from your chemistry text. How many should you know without checking the list? Fair question! Ask your instructor. Try It Out
[Numbers and their Properties] [Numbers in Science] [Ratios and Proportions] [Units, Dimensions, and Conversions] [Percents] [Simple Statistics] [Logarithms] |
 The Shodor Education Foundation, Inc.
The Shodor Education Foundation, Inc.in cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1998
Last Update:
Please direct questions and comments about this page to
WebMaster@shodor.org
