Course Chapters
Section Tests
Calculators Linear Least Squares Regression Newton's Method Equation Solver
|
Another Problem 4Question:If there is originally exactly 1 mole of water (6.022 x 1023 water molecules) in a beaker, and 1,000,000,000. evaporate every millisecond, how many water molecules are remaining after 7.00 days have elapsed?Solution: All of them! Sketch:To do this problem, you must figure out how many milliseconds there are in seven days, and then multiply this number times the number of atoms evaporating. Using the dimensional analysis setup shown below, we determine that we have lost 6.048 x 1017 atoms of water in seven days. If we subtract 6.048 x 1017 from 6.022 x 1023, we get 6.022 x 1023. At least to as many significant digits (3) as you have been given in the problem, the number of atoms would be reported the same. Does your calculator at least indicate some have evaporated? How many would have been left after the first day? The first second? What if you had tried to subtract the number per second -- second after second -- for the whole week? Would your calculator ever have shown a difference? Think about what you learned in the previous lesson about loss of significance.
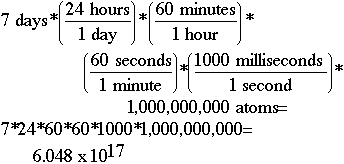
|
 Shodor
Shodorin cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1996-2008 Shodor
Please direct questions and comments about this page to
WebMaster@shodor.org
