Blackbody Radiation
Blackbody RadiationThe color of light produced by a hot object depends on its temperature. This has to do with the fact that more energetic light produced a different wavelength of light. The wavelength, frequency, and energy of light are related by 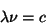
and 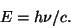
This can be observed visually by considering a traveling wave. Consider a point located in space, being moved up and down as it "sits" on a traveling wave. How often that point oscillates will depend on the wavelength and the wave speed.
Notice how as the wave speed decreases and the wave length increases, the blue dot moves back and forth less often (i.e. has a lower frequency). The amount of radiation given off by a hot object depends on two things, how hot it is, and what wavelength you are observing. The energy per unit area per wavelength is 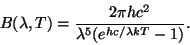
Here h is Planck's constant (6.626E-34 Js), k is Boltzmann's constant (1.381E-23 J/K), and c is the speed of light (2.998E8 m/s). Units are in Watts per square meter per meter. The units make sense here if you think of the total energy given off as the blackbody radiation added up over the entire surface area of the object (Watts per square meter) and then summed up over all the different wavelengths of light (per meter). The curve that this equation gives us changes over a wide range of both intensity and wavelength, and is usually plotted as a log-log plot. When blackbody emmission is observed, while we see light at many wavelengths, there will be one wavelength which is more prevalent than any of the others. This wavelength is approximately 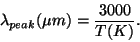
|



