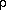 R T
R T
The equation of state for a gas can appear in many forms. One of
the more common forms used by meteorologists looks like this:
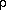 R T
R TThe Javascript calculator to the right will allow you to experiment with the relationships between the variables in this form of the equation of state.
Sample values are supplied to give you a feel for what a "normal" value might be for the variables on the right hang side of the equation. You can go ahead and click on "CALCULATE" to compute the pressure with the default values. Or, by using the "Reset Values", "Clear Field", and "Clear All" buttons, you can solve for any of the variables listed in the table. Just one note, to ensure an accurate computation, be sure to clear the field ("Clear Field") of the variable you wish to solve before you click on "CALCULATE".
The following is a description of each of the variables for which you can solve.
The pressure should be entered in units of kiloPascals (kPA). A Pascal is equal to a kilogram per meter per second squared (kg/m1 s2). While Pa are the standard unit of for pressure, meteorologists usually measure and refer to pressure in terms of kiloPascals (kPa) and millibars (mb). Thus, 1 kPa is equivalent to 1000 Pa and 10 mb. Standard atmospheric pressure at sea level is equal to 1013.25 mb = 101325 Pa = 101.325 kPa.
The density of the gas should be entered in units of (kg/m3) which represent the mass of a measured volume of gas divided by the volume. The standard (accepted average) density of the atmosphere near the surface of the earth is approximately 1.20 kg/m3. But remember, this is only an average; it varies with time and space.
The gas constant is different for every gas. The most common
units for R when making computations are J/kgK
. The two gas constants that meteorologists make frequent use of are the gas constants for dry air (Rd) and pure
water vapor (Rv).
NOTE: Rv, the gas constant for water vapor, is not the
gas constant for moist air. The gas constant for a sample of
moist air varies with the amount of moisture present in the
sample of air. We will discuss this further in this session when
we introduce the concept of virtual
temperature
Rd (dry air) = 461 J/kgK
Rv (water vapor) = 287 J/kgK
The temperature of the gas is generally converted to the Kelvin scale when making computations. However, on this page, in order to save you the trouble of having to convert Celsius temperatures to Kelvin, we have done it for you. Be sure to enter the temperature in degrees Celsius. Refer to the temperature converter if you need to convert from either degrees Fahrenheit or Celsius.