|
|
Ozone, NOx and VOCThe two main chemical species responsible for the formation of ozone are VOC (volatile organic compounds) and NOx (Nitrogen Oxides). NOx are important in that the photolysis of NO2 (10), creates the O that can then combine with O2 to form ozone (11). NO generally forms NO2 through one of the following reactions:RC(O)OO HO2 VOC are important in that their oxidation leads to the formation of radicals, which can then react with NO to form NO2 the following are a few examples of the many oxidation reactions that can occur for VOC: OH R
Aldehyde Oxidation How do VOC and NOx work together?As you can see from the above reactions, VOC and NOx work together to form ozone. VOC provide the radicals to react with NO and form NO2, NOx provide the NO2 and the NO that are crucial in the Carbon Monoxide Oxidation mechanism. Because the key step in the formation of ozone comes from the photolysis of NO2, ozone is formed almost exclusively during the day.But what happens at night?At night, because the NO2 cannot photolyze, there is an excess of NO2 molecules. Some of these molecules are converted to nitric acid (HNO3) through a variety of reactions. The following reaction is an example of one of the many ways to form HNO3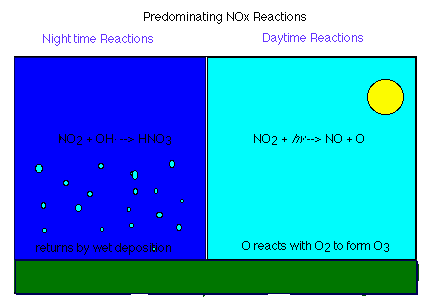
Confused? Have a question? If so, check out the Frequently Asked Questions (FAQ) page or send mail to the OS411 tutor (os411tutor@shodor.org) with your question! Report technical/content problems here |
|
|