|
|
Tropospheric OzoneOzone (O3) is a molecule which can be both beneficial and detrimental to life. In the stratosphere it protects us from harmful ultraviolet radiation, and is essential to life. In the troposphere, however, ozone is a dangerous pollutant, it is a key component in the photochemical smog which effects such cities as Los Angeles and Atlanta. Ironically, there are some human activities which create the dangerous ozone that is increasing in the troposphere, while others destory the ozone of the stratosphere which protects us. A current problem is the reduction of stratospheric ozone due to pollutants such as CFC's (chloroflourocarbons), causing the emergence of an ozone "hole" around Antarctica. Ozone is considered to be a secondary pollutant, as it is not directly emitted into the atmosphere, but is a result of the reactions of pollutants that are emitted.The reaction to form ozone is:
Two of the substances which are most directly involved in forming ozone are NOx (Nitrogen Oxides) which consist of NO and NO2, and VOC (Volatile Organic Compounds). NOx are derived mainly from the combustion of fuel in cars, VOC come both from fossil fuel combustion and from natural sources such as some trees (isoprenes and pinenes) and decomposition of organic material. Although NOx and VOC are the two main sources of ozone, other important precursors include methane (CH4), Carbon Monoxide, and the ever-important hydroxyl radical (OH
The following chart identifies some of the main sources of pollutants, many of which are precursors to ozone: 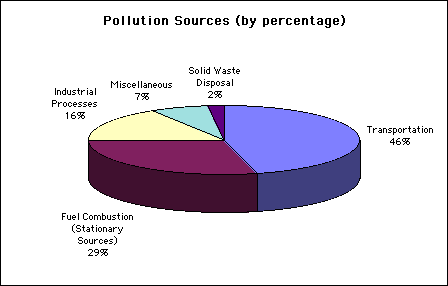
Confused? Have a question? If so, check out the Frequently Asked Questions (FAQ) page or send mail to the OS411 tutor (os411tutor@shodor.org) with your question! Report technical/content problems here |
|
|