|
|
Potential Temperature
Let's suppose you are working outside on a hot summer day. When you go inside to cool off and check out the score of the big game you are missing, a weather alert comes on calling for severe thunderstorms with large damaging hail. How could such large balls of ice fall from up there when it is so hot down here? And if it is cold enough up there to form ice, why can't we just bring some of that cold air down to cool things off a bit?
The equation of state can help us answer these questions. The equation of state helps us figure out the state of the atmosphere under various conditions. For instance, suppose the air at the surface of the earth is dry and in equilibrium, stable, and has a pressure of 1000 mb (100 kPa), a specific volume of 0.83 m3/kg (
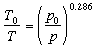 where T0 is the temperature at the higher pressure in degrees Kelvin (K), T is the temperature at the lower pressure in degrees Kelvin, p0 is the higher pressure, and p is the lower pressure. For the parcel of air described above being lifted to a height where the pressure is 700 mb (70 kPa), the temperature would decrease from 25 C to -4 C. That's cold! To answer our initial questions about hail on a hot day and cold air aloft, the air above is so much colder than the air at the surface because the temperature is in equilibrium with the density and pressure at the height it is residing. So, hail can form on a very hot day because the temperature aloft can be below freezing when the temperature at the surface is quite scorching. But if we were to try to bring that same cold air to the surface, the temperature would increase in order to compensate for the adiabatic compression.
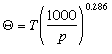 where T is the initial temperature in degrees K and p is the initial pressure in millibars. In addition to calculating potential temperature, adiabatic charts (thermodynamic diagrams) have been constructed which enable us to easily look up the temperature and pressure of a parcel of air and determine its potential temperature. Potential temperature is going to be an important term later when we discuss the vertical stability of the atmosphere. Comparing the potential temperature of a parcel to that of its surroundings can tell us whether a region of air is stable or subject to lifting or sinking. We will see that the issue of stability is a major factor when trying to determine the transport and dispersion of air pollutants. Pollutants trapped in a stable layer can sit stagnant in very high concentrations. Once air becomes unstable and buoyant the air may rise and mix and dilute the pollutants. You can better familiarize yourself with the concept of potential temperature by computing the potential temperatures of air parcels of different pressures and temperatures. This calculator will compute an unknown variable in the equation above given known values for the remaining two variables. Confused? Have a question? If so, check out the Frequently Asked Questions (FAQ) page or send mail to the OS411 tutor (os411tutor@shodor.org) with your question! Report technical/content problems here |
|
|