| |
Enthalpy
The word enthalpy
comes from the Greek "heat inside". If you have a chemical system that
undergoes some kind of change but has a fixed volume, the heat output is
equal to the change in internal energy (q = DE).
We will define the enthalphy change, DH,
of a system as being equal to its heat output at constant pressure:
DH
= q
at constant pressure
You will
not need to calculate the enthalphy directly. Chemists are usually
interested in the change in enthalphy, or DH.
Tables of enthalphies are widely available and can be used to calculate
DH
where,
DH
= DE
+ P D V
DV
is the change of volume, and P is pressure, held at a constant value.
Since work
is defined as PDV,
an enthalphy change is simply the amount of energy change (energy going
in or out, endothermic or exothermic), plus the amount of work being done
by the reaction.
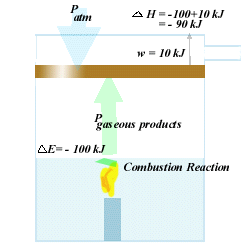
For example,
if DE
= -100 kJ in a combustion of a solid, but 10 kJ of work needs
to be done to move a piston so that there is "space" for the gaseous
products, the change in enthalphy is:
DH
= -100 kJ + 10 kJ = -90 kJ
This is an exothermic
reaction (as you would expect with combustion.), and 90 kJ of energy is
released to the environment.
Notice the convention
used here -- a negative value represents energy coming out of the system
and being transferred to the surroundings.
|
 |
Enthalpy
is a state function. A state function is a property whose
value does not depend on how the change takes place. Your bank balance
is a state function. Your balance of $100 is completely unaffected
by whether you received the money as a gift in one lump some or earned
it for working 10 1-hour shifts. The two paths to the balance are different
but the balance remains the same. The internal energy of a system, E, is
also a state function.
The value
of the state function in determining the change in enthalpy of a system
lies in the fact that it is not necessary to know the exact series of chemical
reactions that transform reactants to products to determine the enthalpy
of a new system. Any series of reactions that gets you from the reactants
to the products will yield the same DH.
Hesse's
Law is a calculating technique that allows you to apply this insight.
The
gases CO and NO are pollutants that form in the exhaust of automobiles.
A chemist studying how to convert them to less harmful gases might start
with the following equation:
CO (g) + NO (g)  CO2 (g) + 1/2 N2 (g)
DH
= ??
CO2 (g) + 1/2 N2 (g)
DH
= ??
She has on
hand the following related information:
Equation A: CO (g) + 1/2O2 (g) CO2 (g) DH
= - 283.0 kJ
CO2 (g) DH
= - 283.0 kJ
Equation B: N2 (g) + O2
(g)  2NO(g)
DH
= 180.6 kJ 2NO(g)
DH
= 180.6 kJ
Perhaps Equations
A and B can be manipulated algebraically to yield the "target" equation,
the one our chemist is working on. Then the DHs
for Equations A & B can be summed to give the correct DH
for the target equation.
reverse Equation
B and divide by 2
NO(g)  1/2 N2 (g) + 1/2O2 (g)
DH
= - 90.3 kJ
1/2 N2 (g) + 1/2O2 (g)
DH
= - 90.3 kJ
CO (g) + 1/2O 2(g)  CO2 (g)
DH
= - 283.0 kJ
CO2 (g)
DH
= - 283.0 kJ
after cancelling the
1/2O 2 that
appears on both sides of the equation and adding the
altered equation Equation B to Equation A, she obtains the target equation and the DH for the target reaction:
NO (g) + CO (g)  CO2 (g) + 1/2 N2 (g)
DH
= - 373.3 kJ
CO2 (g) + 1/2 N2 (g)
DH
= - 373.3 kJ
|
Report technical/Content problems here
| |