| |
LeChatelier's Principle
Le Chatelier's Principle states that if an equilibrium system is disturbed,
it will undergo a reaction that takes it back to equilibrium. Changes
in concentration cause a net reaction away from the added component
toward
the removed component.
You can think of this as being a bit like a child's seesaw. Two children of equal weights might be able to hold the seesaw in perfect balance, but if a heavier child gets on one end, the lighter child might need to add a few rocks in order to restore the balance on the seesaw. If a reaction at equilibrium experiences a change in the
moles of gas, pressure, or volume, a net reaction
that partially reverses the change will occur in order to restore the equilibrium. Although the concentration of
the components changes as a result of the concentration and volume changes,
K, ( the equilibrium constant)
does not change. Nor does a catalyst
affect the equilibrium position because it speeds the reaction equally
in both the forward and reverse directions.
However,a temperature change does change K by
causing a net reaction to replace removed heat (exothermic direction) or
to absorb added heat (endothermic direction). The following table summarizes these affects known as LeChatelier's Principle.
| Change in Concentration |
Change in Volume (Pressure) |
Change in Temperature |
-
K remains the same
-
If concentration of product is increased, then concentration
of reactant must increase to maintain the numerical value of K.
-
As a result, the equilibrium concentration of product is lower
than it was when disturbance occured but concentration of reactants is
higher.
-
Conversely, if reactant concentration is increased,
the equilibrium position moves to the product side and more product is
produced while the concentration of the reactant
is decreased in order to reach equilibrium following the disturbance.
|
-
K remains the same
-
Changing the volume of the container changes the
concentration of the gases in the chamber. When volume is reduced,
pressure is increased and so the concentration increases. The equilibrium
position will shift to relieve the pressure by reducing the number of moles
of gas.
-
If the number of moles of reactant and product are
the same then there will be no effect on equilibrium
|
-
K does not remain the same when the temperature is
changed
-
The direction of the change depends on whether or
not the reaction is exothermic or endothermic.
-
An increase in temperature will cause the equilibrium
of an exothermic reaction to shift towards the reactants.
-
A drop in temperature for the same system would cause
an increase in the product concentration.
|
| Example:
The concentration of H2 is increased
in the following system. Will the conentration of HI increase or
decrease when the new equilibrium is reached?
H2 + I2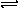 2HI
2HI
Solution: The concentration of HI will increase.
Since K remains the same even after the concentration of hydrogen gas is
increased, the concentration of the product must increase too. The
concentration of hydrogen gas at the new equilibrium will be lower than
it was when more was added but higher than it was at the previous equilibrium. |
Example:
How would you change the volume of the following
system if you wanted to increase the yield of products?
S(s) + 3F2(g) 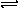 SF6(g)
SF6(g)
Solution:
The solid sulfur does not enter into the calculation
of K for this system. That leaves 3 moles of reactant and 1 mole
of product. Increasing the pressure or reducing the volume of the
container would favor the side with the smaller number of gas moles and
consequently increase the yield. |
Example:
The system
PCl3(g) + Cl2(g) 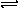 PCl5(g)
PCl5(g)
is exothermic in the forward direction (- delta
Hrxn). How would increasing the temperature affect the equilibrium
concentraton of Cl2(g), and the K?
Solution:
The addition of heat to an exothermic reaction
will favor the reverse reaction so more reactants will be made.
The concentration of chlorine gas will increase. Since the reactants
will be increasing in concentration relative to the products, the numerical
value of K will be smaller. |
For the endothermic reaction: SO2(g)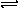 S(s) + O2 S(s) + O2
Report technical/Content problems here
|
|
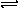 S(s) + O2
S(s) + O2