Equilibrium Concepts
Up to this point we have treated chemical reactions as if, whenever appropriate reactants collide products result. This is often untrue. Many reactions are reversible so that while products are being created, reactants are reforming. The balance between forward and reverse reactions is known as equilibrium. Equilibrium is the Zen topic of chemistry and formal definitions of equilibrium often read like ancient oriental riddles. Equilibrium is reached when the rate of the forward reaction is precisely equal to the rate of the reverse reaction producing the apparently contradictory observation of no change in reactant concentrations, even thought this observable state is present only because both forward and backward reactions are occuring. Equilibrium does not mean that equal concentrations of reactants and products are present. In fact, one of the benefits of understanding equilibrium processes is the ability to predict the amount of product that can be expected for a given reaction and set of reaction conditions. The extent of a reaction --- the concentration of product that is present when no further change is observed --- ultimately depends on the difference in energy between the reactants and the products. The study of the initially contradictory nature of equilibrium and the factors that influence it, have produced some of the most important advances in industrial chemistry. The equilibrium constant is a number based on the ratio of product concentration to reactant concentration. For a reaction of the form:
aA + bB
If most of the reactants go into producing products when the system is at equilibrium then K will be a number larger than 1. If K is very small then very little product is made when equilbrium is reached. The N2O4 - NO2 system provides a unique opportunity to observe an equilibrium process. When dinitrogen tetroxide gas is placed in a sealed container it will decompose to nitrogen dioxide until an equilibrium between the two gases is reached. Dinitrogen tetroxide is a colorless gas but nitrogen dioxide is a dense, deep reddish brown gas so the progess of the reaction towards equilibrium is clearly visible to an observer. The figure below shows this equilibrium system graphically. The box at the top of the figure shows the appearance of the mixture as the reaction proceeds. At the beginning of the experiment the container has only N2O4 in it which is colorless. As more NO2 is made the gas begins to turn light brown and then darkens until equilibrium is reached between the two gases. After equilibrium is reached, the color seems to stabilizes as the rate of formation of NO2 is precisely balanced by the rate of decomposition of NO2 to reform N2O4. 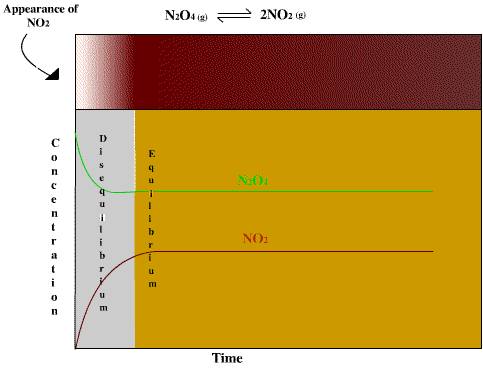
Nitrogen dioxide is a major player in a number of atmospheric chemistry problems. It is an acrid gas that, because of its high density, tends to stay close to the ground. When inhaled, even at retively low concentrations, nitrogen dioxide can cause a variety of acute and chronic respiratory problems. The rate of a reaction and the yield from that reaction are not necessarily related. Kinetics and equilibrium are two distinct aspects of a reacting system. This is simple an interactive model of chemical equilibrium that gives you the opportunity to explore the relationship between the concentrations of reactants and products, and the rates of the forward and reverse reactions. Report technical/Content problems here |
|
|