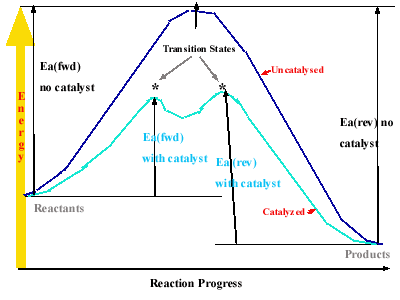
Catalysis
A catalyst is a substance added to a reaction
that speeds up a reaction by making the activation energy lower, which
makes the rate constatn larger and consequently the rate higher.
A catalyst speeds up both the forward and reverse reactions, so that no
more product is made but the reaction yields the product faster.
Catalysts lower the Ea by providing a different mechanism for the reaction
that requires lower energy. The catalyst is not consumed during the
reaction. A catalyst that is in the same phase as reactants is
called a homogeneous catalyst while those that are in a different phase
are referred to as heterogenous catalysts.
Homogeneous catlysis plays a key role in one
of the most serious environmental problems of our time --- stratospheric
ozone depletion. The stratospheric ozone layer blocks out a considerable
portion of the sun's ultraviolet radiation, radiation that can cause serious
health risks to people, animals and plants if it arrive on the earch's
surface without the filtering effect of the ozone layer. Anthropogenic
chlorofluorocarbons (CFC) are released into the atmosphere. When
they reach the troposphere absorb UV photons Cl atoms are released in the
reaction:
CF2Cl2(g) + UV photon   CF2Cl(g)
+ CF2Cl(g)
+  Cl(g) Cl(g)
The dots ( )
represent unpaired electrons resulting from bond breakages. The chlorine
radical is a very reactive species and readily reacts with stratospheric
ozone to produce the intermedicate chlorine monoxide ( )
represent unpaired electrons resulting from bond breakages. The chlorine
radical is a very reactive species and readily reacts with stratospheric
ozone to produce the intermedicate chlorine monoxide ( 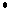 ClO
), that goes on to react with free O atoms to regenerate Cl atoms: ClO
), that goes on to react with free O atoms to regenerate Cl atoms:
O3(g) + 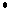 Cl(g) Cl(g)  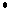 ClO(g)
+ O2(g) ClO(g)
+ O2(g)
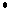 ClO(g)
+ O(g) ClO(g)
+ O(g) 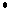 Cl(g)
+ O2(g) Cl(g)
+ O2(g)
The sum of these two elementary reactions gives the overall reaction
ozone breakdown:
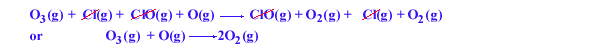
The Cl atom acting as a homogeneous catalyst (since it is in gas phase like the reactants),
speeds up the reaction by introducing a new mechanism. Chlorine gas is regenerated
as part of the final reaction. Because of its ability to regenerate,
a single Cl atom can remain in the stratosphere for about 2 years destroying as many as
100,000 ozone molecules.


Credit: TOMS science team and Scientific Visualization
Studio, NASA GSFC
and Robert Simmons, Research and Professional Services.
NASA Website
| These images were produced by Total Ozone Mapping Spectrometry instruments
aboard NASA's Earth Probe satellite. The image on the left shows the largest
ozone hole detected by NASA over the Antarctic in September 2000.
The figure on the right shows the ozone hole over the antarctic in October
of 2000. The hole expanded to approximately 11 million square
miles in September. The previous record was approximately 10.5 square
miles. Despite cutbacks in the production of CFCs under international
agreements, ozone-destroying gases in the stratospere are only now reaching
their peaks due to their persistence in the atmosphere. |
Report technical/Content problems here
|