Ideal Gas EquationIt follows from the general description of the kinetic theory of gases that pressure, number of atoms of gas, volume and temperature are related parameters. For example, volume is the same for a mole of any gas at the same temperature and pressure. Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. The molar volume of a gas ( volume occupied by 1 mole of gas) can be calculate from:
Since gas pressure is the result from collisions between gas particles and surfaces,
anything that increases the number of collisions per unit
of time will increase pressure. Both increases in temperature and
decreases in volume result in an increase in the number of collisions and
therefore in gas pressure. Increasing the number of gas particles
without any changes in volume or temperature would also result in an increase
in pressure. ideal gas equation..
A simple restatement of this equation, assuming that n, the number of moles of the gas remain constant, makes it possible to calculate P, V or T even when external conditions are changing. Take a look at this sample solution. Remember that STP means standard temperature and pressure, T = 0oC (273 Kelvin) and P = 1 atm. 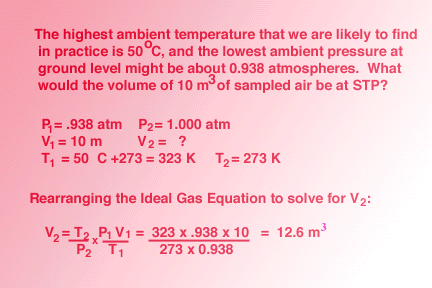 A ideal gas equation calculator is available online. Report technical/Content problems here |
|
|