Hydrogen Bonding
When hydrogen bonding does occur, it greatly affects bulk properties. Liquid water molecules make and break many hydrogen bonds as the molecules move around.
For each molecule, the average number of hydrogen bonds is about 3.4.
When water solidifies molecular movement is slowed. Many of the hydrogen bonds are broken as these molecules form ice crystals.
As a result, the number of hydrogen bonds between molecules is reduced leaving the individual
atoms much farther apart than they were in the liquid state. The
result is a lower density solid than liquid.
These images are taken from the MathMol website where you will find a great deal more information about the water molecule.
Hydrogen bonding significantly
raises the boiling points of liquids in which it occurs. Because
of the increased energy that is necessary to separate hydrogen bonded molecules
from each other, these liquids require much larger amounts of energy to
pass from the liquid to the gaseous phase. 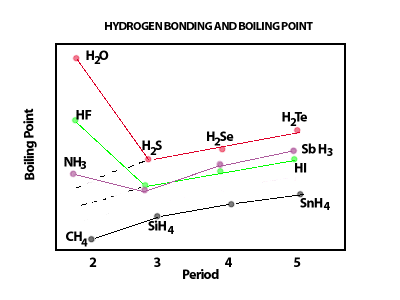 Hydrogen bonding provides a "glue" between the two helices of the DNA molecule. The two strands of the DNA molecule are held together by many hydrogen bonds. Below is a beautiful CHIME rendering of the DNA molecule that will show you the hydrogen bonds as dashed lines. If you see no image below, you will need to install the CHIME plug in. Instructions are provided in the TOOLS pull down menu above. Place your mouse anywhere on the black space around the figure, click and hold. From the menu that appears select "OPTIONS" followed by" DISPLAY HYDROGEN BONDS" |
|
|