| |
The Polar Covalent Bond
While covalent bonds are bonds that result
from the sharing of one or more electron(s), some atoms have a greater
ability to attract electrons in a bond. These atoms are said to be
more electronegative.
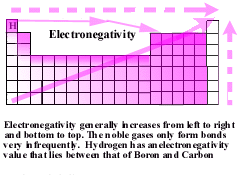
The most electronegative atoms are in the upper right hand corner
of the periodic table, F, and Cl.
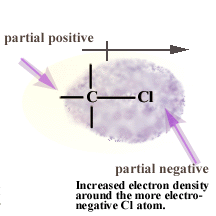 |
Because of chlorine's greater ability
to attract electrons than a carbon atom, the electrons in the covalent
bond between a chlorine atom and a carbon atom are not shared equally. The electron density is greatest near
the most electronegative atom. This creates a nonsymetric electron
cloud with a slight negative charge around the chlorine atom
and a slight positive charge near the more electropositive carbon atom. |
 |
Covalent molecules that form bonds with uneven sharing of electrons
are called polar molecules and
the individual bonds are called polar covalent bonds . The direction of polarity for these molecules is shown using a polarity
arrow,  , with the arrow pointing to the negative pole and the crossed end showing
the positive pole. The same information is sometimes conveyed with
a lower case Greek delta followed by either a plus or a minus sign.
A few compounds are made up of atoms with identical, or near identical, electronegativity values like,
N2. These compounds are held together in nonpolar covalent bonds. , with the arrow pointing to the negative pole and the crossed end showing
the positive pole. The same information is sometimes conveyed with
a lower case Greek delta followed by either a plus or a minus sign.
A few compounds are made up of atoms with identical, or near identical, electronegativity values like,
N2. These compounds are held together in nonpolar covalent bonds.
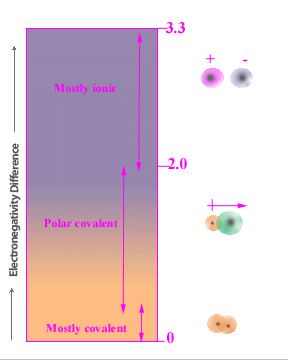 |
Most compounds have bonds that are both ionic and covalent in
nature. The ionic character of
a bond is determined by evaluating electronegativity difference between the two elements in the bond. If that difference is large, then the bond is ionic. An intermediate value for the electronegativity difference would lead to a classification of polar covalent.
When the difference is small, the bond is nonpolar. The electrogativity difference gives the
magnitude and direction of polarity across each bond since the arrow always points to the more negative end of the bond. The vector
sum of all of the bonds is used to determine the overall polarity.
This gives you the polarity of a bond, but how can you determine the polarity for a whole molecule composed of multiple bonds?
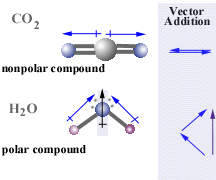
The geometry of the molecule is critical. The water molecule shown below is bent because there are two pairs of
unbonded electrons located on the central oxygen atom. These unbonded electron
pairs or lone pairs repel the
electrons of the hydrogen atoms and force the molecule into a bent position.
The carbon dioxide molecule has no unpaired electrons and so is straight.
As a result of the difference in geometry between these two molecules,
carbon dioxide is nonpolar while water is quite polar. |
Water is perhaps the best known of polar molecules. The polarity
of covalently bonded molecules can lead to a wide range of properties.
For example, covalent compounds that are easily dissolved in water have
at least some polar bonds in them, but, nonpolar compounds, like oils,
do not dissolve in water.
Report technical/Content problems here
|
|
