| |
The Covalent Bond
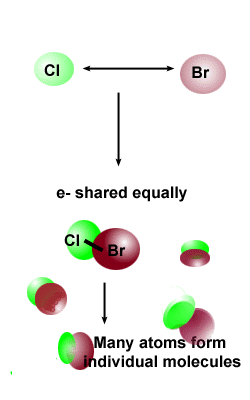
| Covalent compounds are formed when elements share electrons.
They are usually formed between nonmetals Even though
there are relatively few non-metal elements, most compounds, including
all organic compounds, are formed by covalent bonds. The simplest type of covalent bond can be formed
between two hydrogen atoms. The electrons are shared by both nuclei.
Repulsion between the two nuclei and between the electrons also occur,
but the net forces of attraction are greater than the net forces of repulsion. There are six other nonmetal gases that form covalent bonds when they occur
naturally as diatomic gases --- nitrogen, oxygen, fluorine, chlorine,
bromine and iodine. |
 |
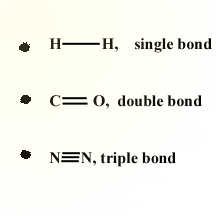 |
Covalent bonds can be single, double, or triple depending upon the number
of bonds each of the atoms involved in the bond can make. In a double
covalent bond, two pairs of electrons are shared. A triple covalent
bond occurs when three electron pairs are shared between two atoms. The bond order, that is single, double or triple bonds, is also an indication of the length
of the bond and the amount of energy that will be released when the bond is formed or
that is required to break the bond. The higher the bond order the
shorter the bond length and the larger the energy involved in making or
breaking the bond |
Report technical/Content problems here
|
|