Chemical BondingIndividual uncombined atoms of elements are rarely encountered in the natural world. Most of the substances around us are combinations of elements bonded together in a relatively limited number of ways. The richness of the natural world is due to the remarkable number compounds that result from about 100 elements binding together to form molecules. Chemical compounds are substances that consists of two or more different elements in a definite ratio. For example, water is a compound made up of two hydrogen atoms each bonded to a single oxygen atom. Sodium chloride, NaCl, is also a compound containing one atom of sodium and one atom of chlorine. Wherever you find water, the ratio of two hydrogen atoms to one oxygen atom will always be true, and table salt will always have a sodium atom in a 1:1 ratio with a chlorine atom. When compounds are not just mixtures of their component atoms. The atoms are actually bonded together and cannot be separated by physical means like distillation, filtering, or with the use of a magnet. In order to return a molecule to its component elements some kind of chemical change needs to occur. When chemical bonding occurs in a diatomic molecule, the resulting compound will always be electonically neutral . It is primarily the electrons of the atoms that are involved in compound formation. Elements combine to form compounds in two general ways:
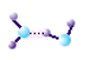 There is another type of chemical "bond" that only occurs between molecules that have hydrogen atoms bonded to
oxygen, nitrogen or fluorine known as hydrogen bonding. Because hydrogen bonding only occurs between molecules,
and does not produce a new molecule, it is fundamentally different from covalent or ionic bonding. There is another type of chemical "bond" that only occurs between molecules that have hydrogen atoms bonded to
oxygen, nitrogen or fluorine known as hydrogen bonding. Because hydrogen bonding only occurs between molecules,
and does not produce a new molecule, it is fundamentally different from covalent or ionic bonding.
A oxygen, nitrogen or fluorine known as molecular formula shows the composition of a molecule in terms of the numbers of atoms of each element present.
Molecules are three dimensional objects that are depicted in a variety of different ways to emphasize some aspect of their formation or behavior. For example, to understand covalent bonding behavior, it is often useful to keep track of the electrons that are available for bonding. Lewis electron dot symbols are a sort of pictorial shorthand that shows what is known about the bonding behavior of an atom. In a Lewis electron dot symbol, the element's symbol represents the nucleus of that atom as well as the inner or core electrons. These are lower energy level electrons. The outer or valence electrons have the highest energy level and generally, they are the electrons that are involved in forming compounds.
Among the main group elements, the valence electrons are the same as the outer electrons, but among the transition element, some inner electrons may be involved in bonding and are counted among the valence electrons. The dots are arranged around the element's symbol to show the characteristic number of bonds for that element. For example, carbon has 4 valence electrons that are arranged singly around the symbol for the element. When a Lewis Structure is drawn to represent a compound there should be 4 pairs of 2 electrons around each atom following the octet rule and consistent with the general principal that individual atoms tend to seek bonds that produce an arrangement of electrons resembling that of the nearest noble gas.
Report technical/Content problems here |
|
|