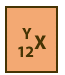| |
Atomic Notation
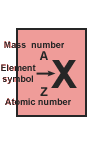 |
The number of protons in the nucleus of an atom is called the atomic number, represented symbolically as Z,
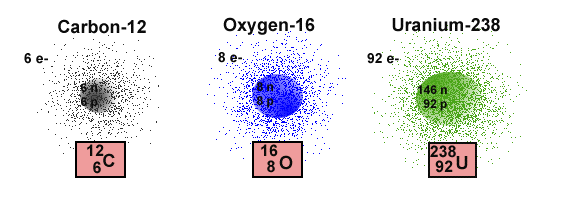
and it is a unique characteristic of an element. Only carbon atoms can
have 6 protons in the nucleus, and only oxygen atoms have a Z=8,
meaning that there are 8 protons in the nucleus of the atom.
The mass of an entire atom can be computed from the the sum of the masses
of the particles in the nucleus. This is possible because the mass
of an electron is vastly smaller than the masses of either the proton or
the neutron. As a result the number of nuclear particles, that is,
n
+ p, can be used to identify the relative mass of any atom, while providing
some additional information about the structure of the atom. This
number, the sum of the neutrons and protons in the atom, is so useful in chemistry
that it has been assigned a special name, mass number, and its own symbol, A. |
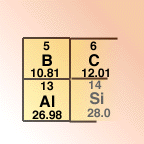 |
Most Periodic Tables
show the atomic number above the symbol for the element, while the
relative mass in units of grams per mole are shown below the element's
symbol. However, in written documents, the number of protons (also
Z and the atomic number are shown as a subscripted prefix of the elemental
symbol. Then the number of nucleons (also referred to as A, and, n + p), are shown as a superscripted prefix. So
neon with 10 protons and 10 neutrons would be shown as : 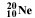 .
You may also encounter several other versions of this format used in print
medium where subscripts and superscripts are not easily available, for
example, Ne-20 or Neon-20. .
You may also encounter several other versions of this format used in print
medium where subscripts and superscripts are not easily available, for
example, Ne-20 or Neon-20. |
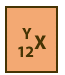
Report technical/Content problems here
|
|