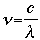
Course Chapters
Section Tests
Online Calculators Linear Least Squares Regression Newton's Method Equation Solver
Related Information Links
|
Problem 1 SolutionUsing the equation:
where:
v = frequency (in units of "per second", or s-1 Solving for frequency, we have the equation:
To ensure that our units match, we must convert the wavelength from nanometers to meters: 1.25 x 103 nm * (1 x 10-9 meters / 1 nm) = 1.25 x 10-6 m Now, it's just a simple "plug and chug": v = 3.00 x 108 m s-1 / 1.25 x 10-6 m = 2.40 x 1014 s-1 or 2.40 x 1014 s-1 hertz (Hz) |
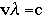
 = wavelength in units of nanometers (nm)
= wavelength in units of nanometers (nm)