Course Chapters
Section Tests
Calculators Linear Least Squares Regression Newton's Method Equation Solver
|
Another Problem 4Question:In chemistry, we can calculate the amount of a chemical produced by a reaction (these calculations are called stoichiometric calculations). The calculation almost always involves figuring how many moles of the reactants (original ingredients) were used given the amount used. This is just a unit conversion problem! Suppose we take iron ore and add it to 1.20 liters of aqueous potassium permanganate to start a reaction. How many moles of the potassium permanganate did we use if we know that 1 liter of the stuff is equivalent to .0195 moles?Solution:
.0234 moles Don't let all the technical terms get in the way; we have 1.2 liters of stuff to convert to moles:
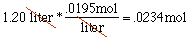 Try another problem like this one. |
 The Shodor Education Foundation, Inc.
The Shodor Education Foundation, Inc.in cooperation with the Department of Chemistry,
The University of North Carolina at Chapel Hill
Copyright © 1998
Last Update:
Please direct questions and comments about this page to
WebMaster@shodor.org
